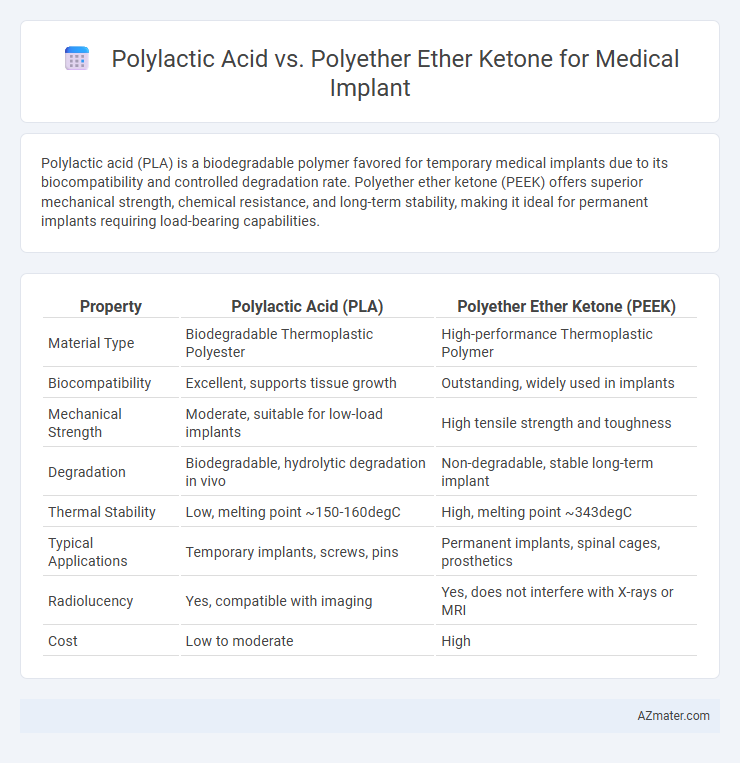
Polylactic acid (PLA) is a biodegradable polymer favored for temporary medical implants due to its biocompatibility and controlled degradation rate. Polyether ether ketone (PEEK) offers superior mechanical strength, chemical resistance, and long-term stability, making it ideal for permanent implants requiring load-bearing capabilities.
Table of Comparison
| Property | Polylactic Acid (PLA) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Material Type | Biodegradable Thermoplastic Polyester | High-performance Thermoplastic Polymer |
| Biocompatibility | Excellent, supports tissue growth | Outstanding, widely used in implants |
| Mechanical Strength | Moderate, suitable for low-load implants | High tensile strength and toughness |
| Degradation | Biodegradable, hydrolytic degradation in vivo | Non-degradable, stable long-term implant |
| Thermal Stability | Low, melting point ~150-160degC | High, melting point ~343degC |
| Typical Applications | Temporary implants, screws, pins | Permanent implants, spinal cages, prosthetics |
| Radiolucency | Yes, compatible with imaging | Yes, does not interfere with X-rays or MRI |
| Cost | Low to moderate | High |
Introduction: Overview of PLA and PEEK in Medical Implants
Polylactic acid (PLA) and polyether ether ketone (PEEK) are prominent materials used in medical implants, each offering distinct advantages in biocompatibility and mechanical properties. PLA is a biodegradable polymer derived from renewable resources, favored for temporary implants that safely degrade in the body, making it ideal for applications such as sutures and scaffolds. PEEK, a high-performance engineering thermoplastic, exhibits superior strength, chemical resistance, and radiolucency, which makes it suitable for long-term, load-bearing orthopedic and dental implants.
Material Composition and Properties Comparison
Polylactic acid (PLA) is a biodegradable aliphatic polyester derived from renewable resources like corn starch, offering excellent biocompatibility and controlled degradation rates ideal for temporary medical implants. Polyether ether ketone (PEEK) is a high-performance thermoplastic with an aromatic backbone, providing exceptional mechanical strength, chemical resistance, and long-term stability suitable for permanent implants. PLA's lower melting point and hydrolytic degradation contrast with PEEK's high melting point and inertness, affecting their respective applications in resorbable versus durable medical device implants.
Biocompatibility: Safety and Immune Response
Polylactic acid (PLA) demonstrates excellent biocompatibility with favorable degradation profiles, minimizing toxic by-products and eliciting mild inflammatory responses that are well-tolerated in medical implants. Polyether ether ketone (PEEK) exhibits exceptional chemical stability and inertness, resulting in minimal immune reaction and stable long-term integration with tissues without degradation concerns. Both materials are widely used in medical implants, but PLA's biodegradable nature offers advantages in temporary scaffolds, whereas PEEK's durability suits permanent implants requiring long-term biostability.
Mechanical Strength and Durability Analysis
Polyether ether ketone (PEEK) exhibits superior mechanical strength and durability compared to polylactic acid (PLA) for medical implants, with a tensile strength ranging from 90 to 100 MPa and exceptional resistance to fatigue and wear. PLA, a biodegradable polymer, demonstrates lower mechanical strength around 50 to 70 MPa and degrades over time, affecting long-term implant stability. The durability of PEEK in physiological environments ensures sustained structural integrity, making it preferable for load-bearing implant applications.
Radiolucency and Imaging Compatibility
Polylactic acid (PLA) exhibits excellent radiolucency, allowing clear visualization and minimal interference in X-ray and MRI imaging, making it ideal for medical implants requiring frequent diagnostic monitoring. Polyether ether ketone (PEEK) offers superior mechanical strength but is less radiolucent, often necessitating imaging markers to distinguish implants during radiographic assessment. Radiolucency in PLA improves compatibility with advanced imaging techniques, enhancing post-operative evaluation and reducing diagnostic artifacts compared to PEEK-based implants.
Degradability and Resorption Profile
Polylactic acid (PLA) exhibits excellent biodegradability and predictable resorption profiles, making it highly suitable for temporary medical implants that require gradual absorption by the body. In contrast, Polyether ether ketone (PEEK) is a non-degradable, highly stable thermoplastic used for permanent implants where long-term mechanical strength and biocompatibility are critical. The choice between PLA and PEEK depends on clinical needs, with PLA favored for resorbable applications and PEEK preferred for durable, long-lasting implant solutions.
Manufacturing Techniques and Customization
Polylactic acid (PLA) is often processed using additive manufacturing techniques like 3D printing, enabling high customization for patient-specific medical implants due to its biodegradability and ease of shaping. In contrast, Polyether ether ketone (PEEK) requires advanced methods such as injection molding and CNC machining to achieve precise geometries, offering superior mechanical strength and chemical resistance but limited flexibility in rapid customization. The choice between PLA and PEEK in medical implants hinges on the balance between manufacturing complexity, customization capability, and material performance requirements.
Clinical Applications: PLA vs PEEK Use Cases
Polylactic acid (PLA) is widely used in biodegradable medical implants such as screws, pins, and scaffolds for tissue engineering due to its bioresorbable properties and compatibility with bone regeneration. Polyether ether ketone (PEEK) is favored in load-bearing, long-term implants like spinal cages and cranial plates because of its superior mechanical strength, chemical resistance, and radiolucency. Clinical applications demonstrate PLA's advantage in temporary implants that promote natural healing, whereas PEEK excels in permanent solutions requiring durability and biocompatibility under physiological stress.
Regulatory Approvals and Standards
Polylactic acid (PLA) is widely recognized for medical implants due to its biodegradability and has FDA clearance under 21 CFR 878.3300 for resorbable polymer devices, ensuring compliance with stringent biocompatibility standards outlined in ISO 10993. Polyether ether ketone (PEEK) meets regulatory approvals such as FDA 510(k) clearances and conforms to ASTM F2026 and ISO 10993 standards, offering excellent mechanical strength and chemical resistance suitable for long-term implant applications.
Future Trends and Innovations in Implant Materials
Polylactic acid (PLA) is gaining traction as a biodegradable, bioresorbable material ideal for temporary medical implants, promoting tissue regeneration and reducing long-term complications. Polyether ether ketone (PEEK) offers superior mechanical strength, chemical resistance, and biocompatibility, making it a preferred choice for long-term, load-bearing implants. Future trends emphasize the development of hybrid composites combining PLA and PEEK to leverage biodegradability with enhanced durability, alongside innovations in surface modification and 3D printing to customize implants tailored to patient-specific anatomical and physiological needs.
Infographic: Polylactic acid vs Polyether ether ketone for Medical Implant
 azmater.com
azmater.com