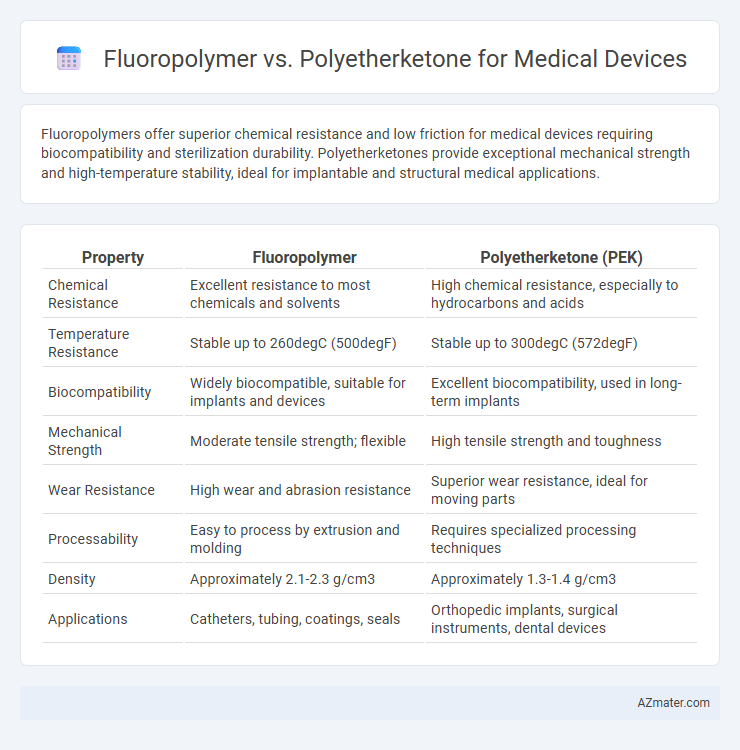
Fluoropolymers offer superior chemical resistance and low friction for medical devices requiring biocompatibility and sterilization durability. Polyetherketones provide exceptional mechanical strength and high-temperature stability, ideal for implantable and structural medical applications.
Table of Comparison
| Property | Fluoropolymer | Polyetherketone (PEK) |
|---|---|---|
| Chemical Resistance | Excellent resistance to most chemicals and solvents | High chemical resistance, especially to hydrocarbons and acids |
| Temperature Resistance | Stable up to 260degC (500degF) | Stable up to 300degC (572degF) |
| Biocompatibility | Widely biocompatible, suitable for implants and devices | Excellent biocompatibility, used in long-term implants |
| Mechanical Strength | Moderate tensile strength; flexible | High tensile strength and toughness |
| Wear Resistance | High wear and abrasion resistance | Superior wear resistance, ideal for moving parts |
| Processability | Easy to process by extrusion and molding | Requires specialized processing techniques |
| Density | Approximately 2.1-2.3 g/cm3 | Approximately 1.3-1.4 g/cm3 |
| Applications | Catheters, tubing, coatings, seals | Orthopedic implants, surgical instruments, dental devices |
Introduction to Fluoropolymers and Polyetherketone in Medical Devices
Fluoropolymers such as PTFE, FEP, and PFA are prized in medical devices for their exceptional chemical resistance, low friction, and biocompatibility, making them ideal for catheters, tubing, and implantable components. Polyetherketone (PEK), including PEEK and PEKK, offers superior mechanical strength, high temperature resistance, and radiolucency, suitable for orthopedic implants, dental devices, and surgical instruments. Both materials support sterilization processes and exhibit excellent durability, but their selection depends on specific device requirements such as flexibility, wear resistance, and regulatory compliance.
Chemical Structure and Material Properties
Fluoropolymers feature carbon-fluorine bonds that provide exceptional chemical resistance, low surface energy, and excellent biocompatibility, making them ideal for medical devices requiring inert and non-reactive surfaces. Polyetherketones (PEK), characterized by aromatic rings linked by ether and ketone groups, offer high thermal stability, mechanical strength, and resistance to radiation and wear, suitable for implantable applications needing structural durability. The chemical structure of fluoropolymers promotes superior hydrophobicity and flexibility, while polyetherketones deliver rigidity and toughness, guiding material selection based on the specific medical device performance requirements.
Biocompatibility and Toxicity Profiles
Fluoropolymers exhibit exceptional biocompatibility due to their chemical inertness, low surface energy, and resistance to protein adsorption, making them ideal for blood-contacting medical devices with minimal immune response. Polyetherketone (PEK), including PEEK variants, demonstrates high biostability and structural integrity under physiological conditions but may require surface modifications to enhance tissue integration and minimize inflammatory reactions. Both materials have low toxicity profiles; however, fluoropolymers' superior resistance to chemical degradation reduces the risk of leachable toxic by-products compared to polyetherketones.
Mechanical Strength and Durability Comparison
Fluoropolymers exhibit exceptional chemical resistance and low friction, but their mechanical strength and durability under high stress conditions are generally lower compared to polyetherketones. Polyetherketones, including PEEK, offer superior tensile strength and fatigue resistance, making them more suitable for load-bearing medical devices requiring long-term stability. The enhanced thermal stability and wear resistance of polyetherketones ensure consistent performance in demanding medical applications where mechanical reliability is critical.
Sterilization Resistance and Reprocessing Capabilities
Fluoropolymers exhibit superior sterilization resistance due to their excellent chemical inertness and thermal stability, making them ideal for repeated autoclaving and gamma radiation sterilization in medical devices. Polyetherketones (PEK), while offering high mechanical strength and good chemical resistance, may experience degradation or diminished mechanical properties after prolonged exposure to aggressive sterilization processes such as steam or ethylene oxide. The reprocessing capabilities of fluoropolymers support extended device lifespan with minimal material compromise, whereas PEK materials require careful assessment of sterilization cycles to maintain device integrity over multiple reuses.
Chemical and Thermal Stability in Medical Settings
Fluoropolymers exhibit superior chemical resistance against acids, bases, and solvents, making them ideal for harsh sterilization processes in medical device applications. Polyetherketones, known for their exceptional thermal stability, maintain structural integrity and mechanical strength at continuous use temperatures exceeding 260degC. The combination of fluoropolymer's chemical inertness and polyetherketone's high-temperature endurance ensures reliable performance and longevity in demanding medical environments.
Applications in Medical Devices: Use Cases
Fluoropolymers are widely used in medical devices due to their exceptional chemical resistance and low friction properties, ideal for catheter coatings, guidewires, and implantable devices requiring biocompatibility and lubricity. Polyetherketone (PEEK) offers superior mechanical strength, thermal stability, and radiolucency, making it suitable for orthopedic implants, spinal cages, and dental devices where durability and imaging compatibility are critical. Both materials enhance patient safety and device performance across diverse applications in minimally invasive surgery and long-term implantable devices.
Regulatory Approvals and Compliance Requirements
Fluoropolymers, such as PTFE and FEP, benefit from extensive FDA approvals and biocompatibility certifications, facilitating their widespread use in catheter coatings and implantable devices. Polyetherketones (PEK), including PEEK, offer superior mechanical strength and thermal stability with increasing FDA clearances for permanent implants, meeting ISO 10993 biocompatibility standards. Regulatory compliance for both materials requires rigorous testing for cytotoxicity, sterilization compatibility, and chemical resistance to ensure patient safety and device efficacy.
Cost Analysis and Manufacturing Considerations
Fluoropolymers, such as PTFE and FEP, offer superior chemical resistance and biocompatibility, but their high raw material and processing costs elevate overall manufacturing expenses compared to polyetherketones like PEEK, which provide excellent mechanical strength and thermal stability at a moderate price point. Manufacturing fluoropolymers requires specialized equipment for high-temperature sintering and extrusion, increasing production complexity and lead times, whereas polyetherketones benefit from established injection molding and machining techniques that streamline volume production. Cost analysis reveals that while fluoropolymers are ideal for applications demanding extreme inertness, polyetherketones present a more cost-effective solution for durable, high-performance medical devices with scalable manufacturing capabilities.
Future Trends in Fluoropolymer and Polyetherketone Medical Applications
Fluoropolymers and polyetherketones (PEK) are increasingly favored in medical devices due to their exceptional chemical resistance, biocompatibility, and thermal stability, enabling advanced implantable and minimally invasive technologies. Future trends highlight fluoropolymers incorporating enhanced surface modifications for antimicrobial properties and improved drug delivery systems, while polyetherketones are evolving with reinforced composites and additive manufacturing to optimize mechanical strength and customizability. Continued innovation in polymer blends and nanocomposites is driving the expansion of their applications in cardiovascular, orthopedic, and diagnostic devices, promising higher performance and patient outcomes.
Infographic: Fluoropolymer vs Polyetherketone for Medical Device
 azmater.com
azmater.com