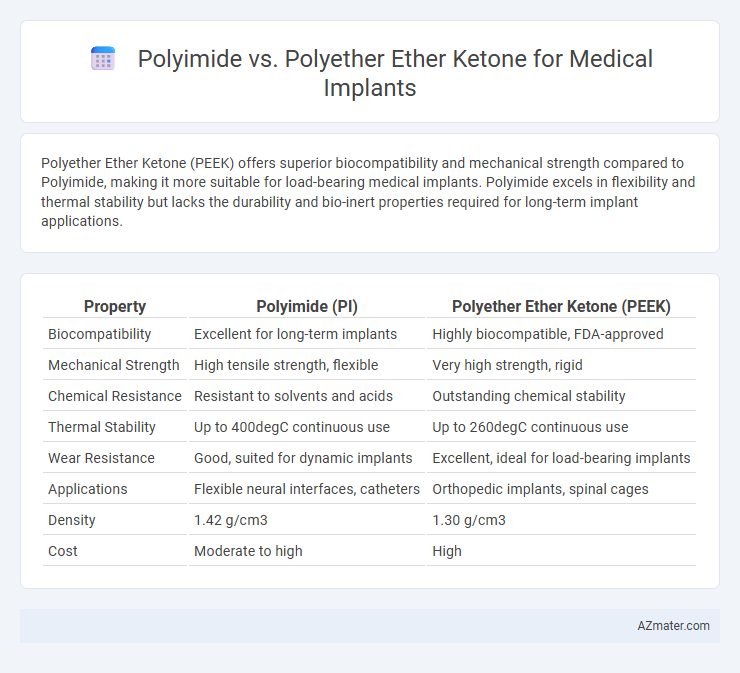
Polyether Ether Ketone (PEEK) offers superior biocompatibility and mechanical strength compared to Polyimide, making it more suitable for load-bearing medical implants. Polyimide excels in flexibility and thermal stability but lacks the durability and bio-inert properties required for long-term implant applications.
Table of Comparison
| Property | Polyimide (PI) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Biocompatibility | Excellent for long-term implants | Highly biocompatible, FDA-approved |
| Mechanical Strength | High tensile strength, flexible | Very high strength, rigid |
| Chemical Resistance | Resistant to solvents and acids | Outstanding chemical stability |
| Thermal Stability | Up to 400degC continuous use | Up to 260degC continuous use |
| Wear Resistance | Good, suited for dynamic implants | Excellent, ideal for load-bearing implants |
| Applications | Flexible neural interfaces, catheters | Orthopedic implants, spinal cages |
| Density | 1.42 g/cm3 | 1.30 g/cm3 |
| Cost | Moderate to high | High |
Introduction to Polyimide and Polyether Ether Ketone in Medical Implants
Polyimide exhibits exceptional thermal stability, chemical resistance, and mechanical strength, making it ideal for flexible, biocompatible medical implants requiring electrical insulation. Polyether ether ketone (PEEK) offers high mechanical strength, radiolucency, and biocompatibility, commonly used in load-bearing orthopedic and spinal implants. Both polymers provide unique advantages for medical implants based on specific application demands, with Polyimide suited for flexible devices and PEEK preferred for durable, structural components.
Material Composition and Chemical Structure
Polyimide consists of aromatic polyimide chains featuring imide groups that provide excellent thermal stability and chemical resistance, making it suitable for medical implants exposed to harsh environments. Polyether Ether Ketone (PEEK) is a semi-crystalline thermoplastic composed of repeating ether and ketone groups, offering exceptional mechanical strength, biocompatibility, and resistance to bodily fluids. The distinct chemical structures define their performance: polyimide's imide bonds ensure durability at high temperatures, while PEEK's ketone and ether linkages contribute to its toughness and chemical inertness in implant applications.
Mechanical Properties Comparison
Polyether Ether Ketone (PEEK) exhibits superior mechanical strength and stiffness compared to Polyimide, making it more suitable for load-bearing medical implants. PEEK's high tensile strength, typically around 90-100 MPa, and its excellent fatigue resistance ensure long-term durability under physiological conditions. In contrast, Polyimide offers flexibility and thermal stability but generally demonstrates lower mechanical strength and modulus, limiting its use in applications requiring rigid structural support.
Biocompatibility and Cytotoxicity
Polyimide and Polyether Ether Ketone (PEEK) are widely evaluated for medical implants, with PEEK demonstrating superior biocompatibility due to its inert surface and minimal inflammatory response in vivo. Studies indicate that PEEK exhibits lower cytotoxicity compared to polyimide, attributed to its stable chemical structure and resistance to degradation in physiological environments. The FDA has approved PEEK for long-term implant use, highlighting its favorable interaction with human tissues and enhanced safety profile over polyimide in biomedical applications.
Sterilization Resistance and Methods
Polyether Ether Ketone (PEEK) exhibits superior sterilization resistance compared to Polyimide, maintaining its mechanical and chemical integrity under repeated autoclaving and gamma irradiation, which are common sterilization methods for medical implants. Polyimide tends to degrade or discolor when exposed to harsh sterilization techniques such as steam sterilization and gamma radiation, limiting its long-term use in implantable devices. For sterilization, PEEK is compatible with autoclaving, ethylene oxide gas (EtO), gamma irradiation, and electron beam sterilization, making it a preferred choice in demanding medical environments.
Long-Term Durability in Biological Environments
Polyether Ether Ketone (PEEK) exhibits superior long-term durability in biological environments compared to Polyimide, primarily due to its exceptional chemical resistance and biostability under physiological conditions. PEEK maintains mechanical integrity and resists hydrolysis and oxidative degradation over extended implantation periods, which is critical for medical implant applications requiring sustained performance. In contrast, Polyimide's susceptibility to moisture absorption and potential degradation limits its long-term reliability in vivo, making PEEK the preferred polymer for durable, high-performance medical implants.
Imaging Compatibility: MRI and Radiolucency
Polyether ether ketone (PEEK) exhibits superior imaging compatibility compared to polyimide, providing excellent MRI safety with minimal artifact formation due to its radiolucent properties. Polyimide tends to produce more interference during MRI scans because of its higher magnetic susceptibility and less radiolucency on X-rays, complicating diagnostic imaging. PEEK's low magnetic susceptibility and high radiolucency make it preferable for medical implants requiring clear MRI visualization and accurate radiographic assessments.
Fabrication Techniques for Medical Implants
Polyimide and Polyether Ether Ketone (PEEK) differ significantly in fabrication techniques for medical implants, with polyimide often processed via spin coating and photolithography for flexible electronics and thin-film applications, while PEEK is typically shaped through machining, injection molding, and extrusion for load-bearing implants. The high thermal stability and chemical resistance of PEEK allow it to maintain mechanical integrity during sterilization, making it ideal for complex, durable medical devices. Polyimide's superior flexibility and biocompatibility enable its use in microfabricated implants, but its processing requires controlled curing environments to achieve optimal performance in biomedical settings.
Regulatory Approvals and Clinical Applications
Polyether Ether Ketone (PEEK) holds a significant advantage over Polyimide in regulatory approvals for medical implants, owing to its long-standing FDA clearance and widespread use in spinal and orthopedic devices. PEEK's biocompatibility and mechanical properties have been extensively validated in clinical applications, including load-bearing implants and cranial prostheses. In contrast, Polyimide's limited regulatory history and clinical data restrict its current use primarily to non-load-bearing components and experimental devices.
Cost Analysis and Market Trends
Polyether Ether Ketone (PEEK) commands a higher price point than Polyimide due to superior mechanical strength, chemical resistance, and biocompatibility, making it a premium choice for long-term medical implants despite increased material costs. Polyimide offers cost-effectiveness with adequate performance for short-term or less mechanically demanding applications, appealing to budget-sensitive medical device manufacturers. Market trends indicate growing PEEK adoption driven by advances in 3D printing and regulatory approvals, while Polyimide maintains niche usage in flexible implants and electronic components, balancing cost and functionality.
Infographic: Polyimide vs Polyether Ether Ketone for Medical Implant
 azmater.com
azmater.com