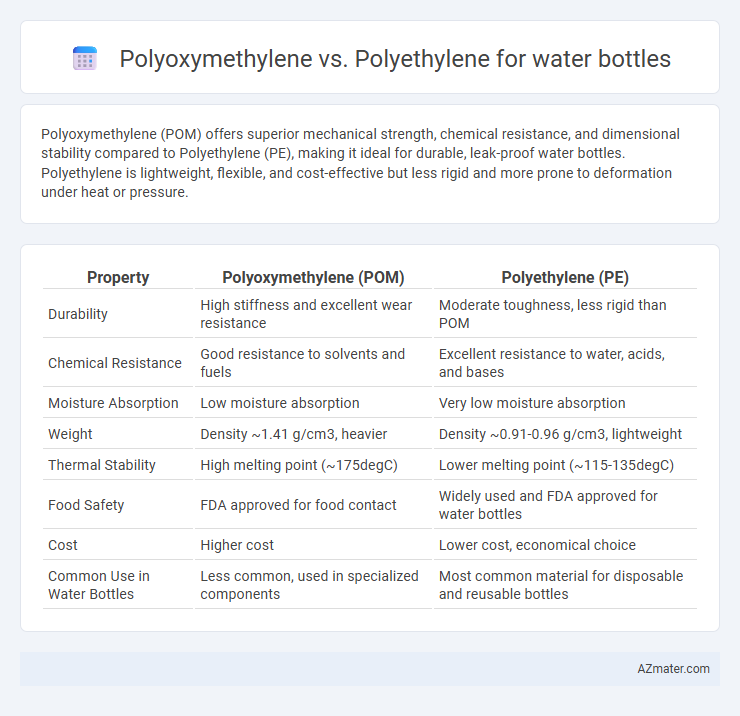
Polyoxymethylene (POM) offers superior mechanical strength, chemical resistance, and dimensional stability compared to Polyethylene (PE), making it ideal for durable, leak-proof water bottles. Polyethylene is lightweight, flexible, and cost-effective but less rigid and more prone to deformation under heat or pressure.
Table of Comparison
| Property | Polyoxymethylene (POM) | Polyethylene (PE) |
|---|---|---|
| Durability | High stiffness and excellent wear resistance | Moderate toughness, less rigid than POM |
| Chemical Resistance | Good resistance to solvents and fuels | Excellent resistance to water, acids, and bases |
| Moisture Absorption | Low moisture absorption | Very low moisture absorption |
| Weight | Density ~1.41 g/cm3, heavier | Density ~0.91-0.96 g/cm3, lightweight |
| Thermal Stability | High melting point (~175degC) | Lower melting point (~115-135degC) |
| Food Safety | FDA approved for food contact | Widely used and FDA approved for water bottles |
| Cost | Higher cost | Lower cost, economical choice |
| Common Use in Water Bottles | Less common, used in specialized components | Most common material for disposable and reusable bottles |
Introduction to Polyoxymethylene and Polyethylene
Polyoxymethylene (POM), also known as acetal, is a high-performance engineering thermoplastic characterized by its excellent mechanical strength, stiffness, and low friction, making it suitable for durable water bottle components. Polyethylene (PE), commonly used for water bottles, is a versatile, lightweight polymer with good chemical resistance and impact strength, available in various densities such as HDPE and LDPE. The choice between Polyoxymethylene and Polyethylene for water bottles involves balancing POM's superior rigidity and wear resistance against PE's cost-effectiveness and flexibility.
Chemical Structure Comparison
Polyoxymethylene (POM) is a highly crystalline thermoplastic with a repeating -(CH2-O)- backbone, offering excellent dimensional stability and chemical resistance ideal for water bottle components. Polyethylene (PE), composed of long chains of -(CH2-CH2)- units, is less rigid and more chemically inert but has lower mechanical strength compared to POM. The key structural difference, an ether oxygen atom within POM's backbone versus the simple hydrocarbon chains of PE, contributes to POM's superior stiffness and resistance to solvents commonly found in water bottle applications.
Physical Properties of POM and PE
Polyoxymethylene (POM) exhibits high tensile strength, excellent rigidity, and superior dimensional stability compared to polyethylene (PE), making it highly resistant to deformation under stress. POM also offers low moisture absorption and excellent chemical resistance, which enhance its durability in water bottle applications. Polyethylene, while more flexible and impact-resistant, has a lower melting point and higher permeability, which can affect the bottle's structural integrity and shelf life.
Durability and Strength Analysis
Polyoxymethylene (POM) exhibits higher tensile strength and rigidity compared to polyethylene (PE), making it more suitable for durable water bottles that require resistance to impact and wear. Polyethylene, especially high-density polyethylene (HDPE), offers excellent flexibility and chemical resistance but has lower tensile strength, which may limit its performance under heavy mechanical stress. For applications demanding long-term durability and structural integrity, Polyoxymethylene stands out due to its superior mechanical properties and resistance to creep deformation.
Chemical Resistance in Water Bottle Applications
Polyoxymethylene (POM) exhibits superior chemical resistance compared to polyethylene (PE) in water bottle applications, effectively resisting degradation from acids, bases, and solvents commonly found in beverages. POM's high crystallinity and strong molecular bonds prevent leaching and maintain structural integrity under prolonged contact with water and cleaning agents. In contrast, polyethylene, especially low-density PE, shows lower resistance to chemical attack and can absorb substances, potentially compromising water purity and bottle durability.
Safety and Food Contact Regulations
Polyoxymethylene (POM) offers high mechanical strength and chemical resistance but has limited approval for direct food contact under FDA and EFSA regulations, restricting its use in water bottles. Polyethylene (PE), particularly high-density polyethylene (HDPE), is widely recognized for food safety compliance, including FDA 21 CFR 177.1520, making it a preferred material for water bottles due to its excellent chemical inertness and non-toxicity. Safety concerns with POM arise from potential formaldehyde release during degradation, whereas PE's proven biocompatibility and non-leaching properties ensure safer water storage.
Environmental Impact and Recyclability
Polyoxymethylene (POM) offers high durability and chemical resistance, but its environmental impact is higher due to slower biodegradation and limited recycling infrastructure compared to polyethylene (PE). Polyethylene, commonly used in water bottles, supports a more established recycling stream and lower carbon footprint throughout its lifecycle, making it a more eco-friendly option. The recyclability of PE, especially HDPE grade, enhances its environmental sustainability in water bottle production.
Manufacturing Process Differences
Polyoxymethylene (POM) is produced through polymerization of formaldehyde, involving precise control of temperature and catalysts in a step-growth process, resulting in a crystalline, high-strength polymer ideal for water bottle components requiring rigidity and chemical resistance. Polyethylene (PE), typically manufactured via chain-growth polymerization of ethylene monomers using catalysts like Ziegler-Natta or metallocene, offers versatility with varying densities (LDPE, HDPE) that influence flexibility and impact resistance in water bottle applications. The manufacturing differences affect molding techniques: POM demands higher processing temperatures and specialized equipment due to its melting point (~175degC), whereas PE's lower melting range (110-140degC) allows for easier extrusion and blow molding, impacting production efficiency and final product properties.
Cost Considerations for Water Bottle Production
Polyethylene is generally more cost-effective than polyoxymethylene for water bottle production due to its widespread availability and lower raw material prices. Polyoxymethylene, while offering superior mechanical properties and chemical resistance, involves higher processing costs and material expenses that increase the overall production cost. Manufacturers often choose polyethylene to optimize budget constraints without compromising basic durability and flexibility required for water bottles.
Conclusion: Best Choice for Water Bottles
Polyethylene is the best choice for water bottles due to its excellent chemical resistance, lightweight properties, and cost-effectiveness, making it ideal for safe, everyday use. Polyoxymethylene, while strong and rigid, is less flexible and more prone to hydrolytic degradation, which limits its suitability for water storage. Therefore, polyethylene's durability, food safety standards compliance, and recyclability position it as the preferred material for water bottle manufacturing.
Infographic: Polyoxymethylene vs Polyethylene for Water bottle
 azmater.com
azmater.com