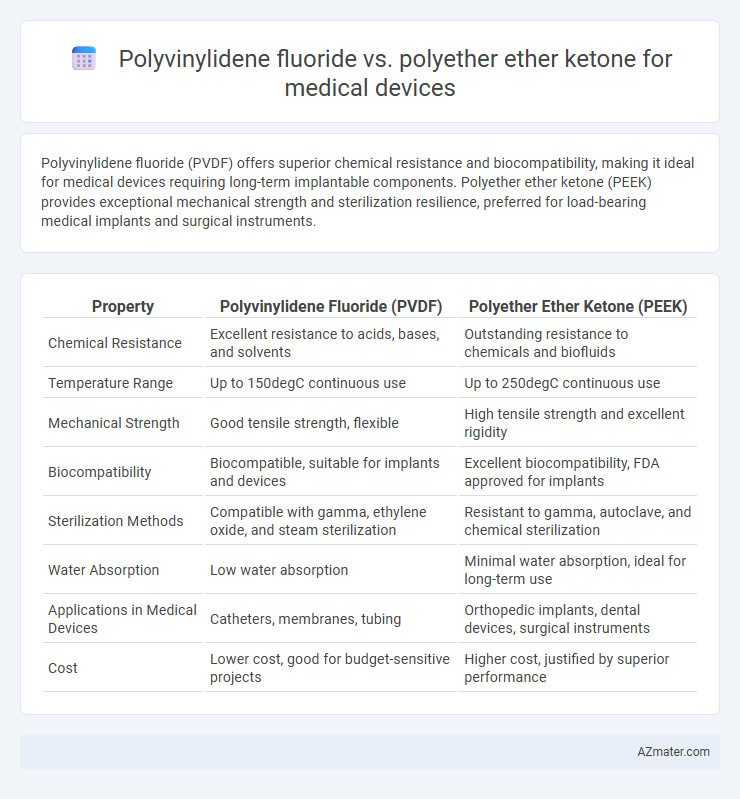
Polyvinylidene fluoride (PVDF) offers superior chemical resistance and biocompatibility, making it ideal for medical devices requiring long-term implantable components. Polyether ether ketone (PEEK) provides exceptional mechanical strength and sterilization resilience, preferred for load-bearing medical implants and surgical instruments.
Table of Comparison
| Property | Polyvinylidene Fluoride (PVDF) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Chemical Resistance | Excellent resistance to acids, bases, and solvents | Outstanding resistance to chemicals and biofluids |
| Temperature Range | Up to 150degC continuous use | Up to 250degC continuous use |
| Mechanical Strength | Good tensile strength, flexible | High tensile strength and excellent rigidity |
| Biocompatibility | Biocompatible, suitable for implants and devices | Excellent biocompatibility, FDA approved for implants |
| Sterilization Methods | Compatible with gamma, ethylene oxide, and steam sterilization | Resistant to gamma, autoclave, and chemical sterilization |
| Water Absorption | Low water absorption | Minimal water absorption, ideal for long-term use |
| Applications in Medical Devices | Catheters, membranes, tubing | Orthopedic implants, dental devices, surgical instruments |
| Cost | Lower cost, good for budget-sensitive projects | Higher cost, justified by superior performance |
Introduction to PVDF and PEEK in Medical Devices
Polyvinylidene fluoride (PVDF) is a highly thermoplastic fluoropolymer known for its excellent chemical resistance, biocompatibility, and mechanical strength, making it ideal for implantable medical devices and fluid-handling components. Polyether ether ketone (PEEK) offers superior mechanical properties, thermal stability, and radiolucency, which are critical for orthopedic implants, spinal devices, and surgical instruments. Both PVDF and PEEK provide sterilization compatibility and long-term durability, positioning them as essential materials in advanced medical device manufacturing.
Chemical Structure and Properties Comparison
Polyvinylidene fluoride (PVDF) features a semi-crystalline structure with repeating -(CH2-CF2)- units, offering excellent chemical resistance and moderate mechanical strength ideal for medical devices exposed to aggressive chemicals. Polyether ether ketone (PEEK) possesses a high-performance aromatic polymer backbone with ketone and ether linkages, providing superior thermal stability, exceptional mechanical properties, and biocompatibility crucial for implantable medical components. The fluorinated nature of PVDF imparts higher chemical inertness, while PEEK's rigid molecular structure ensures enhanced wear resistance and dimensional stability in sterilization processes.
Mechanical Strength and Durability
Polyether ether ketone (PEEK) offers superior mechanical strength and exceptional durability compared to polyvinylidene fluoride (PVDF) in medical devices, making it ideal for load-bearing and high-stress applications. PEEK's high tensile strength, fatigue resistance, and chemical stability under sterilization processes outperform PVDF, which has lower mechanical robustness but better flexibility and chemical resistance. The enhanced wear resistance and thermal stability of PEEK contribute to its preference in long-term implantable devices where mechanical integrity is critical.
Biocompatibility and Safety Profiles
Polyvinylidene fluoride (PVDF) exhibits excellent biocompatibility with high chemical resistance and low protein adsorption, making it suitable for long-term medical device implantation. Polyether ether ketone (PEEK) also offers superior biocompatibility combined with exceptional mechanical strength and thermal stability, widely used in orthopedic implants and spinal devices. Both polymers provide robust safety profiles; however, PEEK's radiolucency and resistance to sterilization methods give it a distinct advantage in applications requiring imaging compatibility and repeat sterilization.
Sterilization Resistance and Process Compatibility
Polyvinylidene fluoride (PVDF) offers exceptional sterilization resistance due to its high chemical inertness, allowing repeated exposure to steam, gamma irradiation, and ethylene oxide without significant degradation. Polyether ether ketone (PEEK) also exhibits excellent sterilization tolerance, maintaining mechanical integrity under autoclave conditions and radiation sterilization, though its higher melting point requires precise processing controls. PVDF's lower processing temperature and chemical resistance make it compatible with injection molding and extrusion, whereas PEEK demands higher temperatures and specialized equipment, influencing device fabrication choices.
Thermal and Chemical Stability
Polyvinylidene fluoride (PVDF) demonstrates exceptional chemical resistance and maintains structural integrity in harsh chemical environments, making it suitable for medical devices exposed to aggressive sterilization processes. Polyether ether ketone (PEEK) offers superior thermal stability with a high melting point around 343degC, allowing continuous use at elevated temperatures without degradation. Both polymers provide excellent chemical inertness, but PEEK's enhanced thermal endurance is preferred for applications requiring repeated high-temperature sterilization cycles.
Applications in Medical Devices
Polyvinylidene fluoride (PVDF) excels in applications requiring chemical resistance and biocompatibility, making it ideal for fluid handling systems, catheters, and dialysis components. Polyether ether ketone (PEEK) is favored for its high mechanical strength, sterilizability, and radiolucency, often used in implantable devices, surgical instruments, and bone fixation devices. Both polymers support critical medical device functions but differ primarily in mechanical properties and application-specific durability.
Manufacturing Flexibility and Machinability
Polyvinylidene fluoride (PVDF) offers superior manufacturing flexibility with ease of extrusion, injection molding, and welding, making it ideal for complex medical device components requiring chemical resistance and sterilization compatibility. Polyether ether ketone (PEEK) demonstrates exceptional machinability with high precision CNC compatibility, enabling intricate designs and tight tolerances essential for critical medical implants and surgical instruments. PVDF's cost-effectiveness and processability contrast with PEEK's higher thermal resistance and mechanical strength, guiding material selection based on specific device manufacturing requirements.
Cost Considerations and Economic Impact
Polyvinylidene fluoride (PVDF) offers a cost advantage for medical devices due to its lower raw material and processing expenses compared to Polyether ether ketone (PEEK), which is significantly more expensive but provides superior mechanical strength and chemical resistance. PVDF's affordability supports budget-sensitive applications, but PEEK's durability and biocompatibility justify its higher upfront costs through enhanced device longevity and reduced maintenance or replacement needs. Economic impact assessments highlight that while PVDF decreases initial expenditure, PEEK may yield better long-term value in critical implantable or high-performance medical devices.
Future Trends and Innovations in Medical Polymers
Polyvinylidene fluoride (PVDF) is gaining traction in medical devices due to its excellent chemical resistance, biocompatibility, and piezoelectric properties, making it ideal for sensors and implantable devices. Polyether ether ketone (PEEK) remains a gold standard for orthopedic implants and surgical instruments because of its superior mechanical strength, thermal stability, and radiolucency. Future trends emphasize the development of hybrid materials combining PVDF's flexibility and PEEK's durability with bioactive coatings and nanoscale modifications to enhance integration, antimicrobial performance, and long-term functionality in advanced medical applications.
Infographic: Polyvinylidene fluoride vs Polyether ether ketone for Medical device
 azmater.com
azmater.com