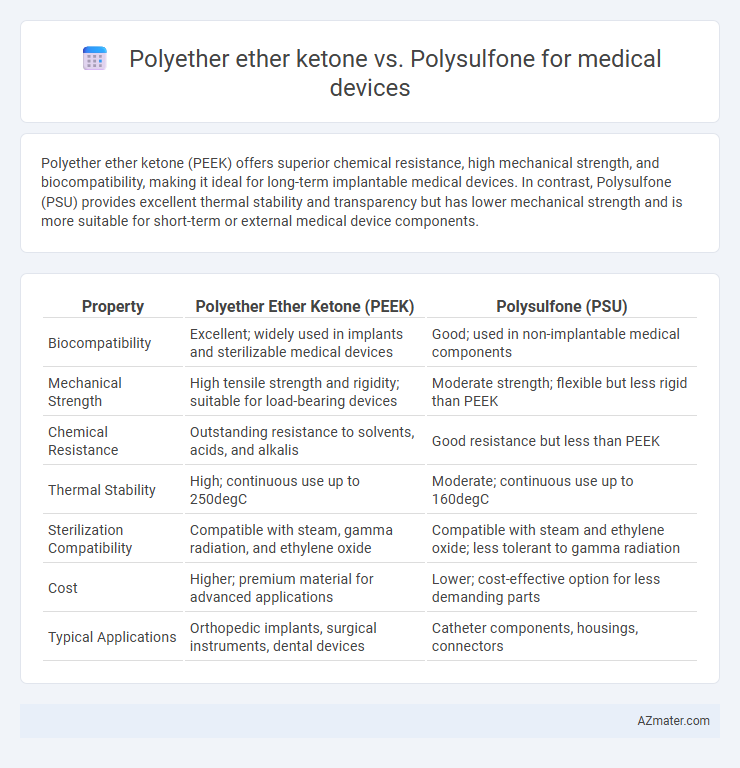
Polyether ether ketone (PEEK) offers superior chemical resistance, high mechanical strength, and biocompatibility, making it ideal for long-term implantable medical devices. In contrast, Polysulfone (PSU) provides excellent thermal stability and transparency but has lower mechanical strength and is more suitable for short-term or external medical device components.
Table of Comparison
| Property | Polyether Ether Ketone (PEEK) | Polysulfone (PSU) |
|---|---|---|
| Biocompatibility | Excellent; widely used in implants and sterilizable medical devices | Good; used in non-implantable medical components |
| Mechanical Strength | High tensile strength and rigidity; suitable for load-bearing devices | Moderate strength; flexible but less rigid than PEEK |
| Chemical Resistance | Outstanding resistance to solvents, acids, and alkalis | Good resistance but less than PEEK |
| Thermal Stability | High; continuous use up to 250degC | Moderate; continuous use up to 160degC |
| Sterilization Compatibility | Compatible with steam, gamma radiation, and ethylene oxide | Compatible with steam and ethylene oxide; less tolerant to gamma radiation |
| Cost | Higher; premium material for advanced applications | Lower; cost-effective option for less demanding parts |
| Typical Applications | Orthopedic implants, surgical instruments, dental devices | Catheter components, housings, connectors |
Introduction to Polyether Ether Ketone (PEEK) and Polysulfone (PSU)
Polyether ether ketone (PEEK) is a high-performance thermoplastic known for its exceptional chemical resistance, mechanical strength, and biocompatibility, making it ideal for demanding medical device applications such as implants and surgical instruments. Polysulfone (PSU) offers good thermal stability, toughness, and hydrolytic resistance, frequently used in medical components requiring sterilization and durability in aqueous environments. Both materials provide distinct advantages in medical device manufacturing, with PEEK favored for load-bearing applications and PSU suitable for devices exposed to repeated sterilization cycles.
Chemical Structure and Material Properties Comparison
Polyether ether ketone (PEEK) features a semi-crystalline polymer structure with repeating ether and ketone groups, providing exceptional chemical resistance, high thermal stability up to 250degC, and superior mechanical strength ideal for durable medical devices. Polysulfone (PSU) is an amorphous polymer containing sulfone and ether linkages, offering excellent hydrolytic stability, transparency, and impact resistance but with lower thermal resistance around 180degC compared to PEEK. The rigid backbone and crystallinity of PEEK result in enhanced wear resistance and sterilization tolerance, while polysulfone's flexibility and clarity make it suitable for components requiring toughness and biocompatibility without extreme heat exposure.
Biocompatibility and Safety in Medical Applications
Polyether ether ketone (PEEK) exhibits superior biocompatibility and chemical resistance compared to polysulfone (PSU), making it highly suitable for medical devices requiring long-term implantation. PEEK's inert nature minimizes inflammatory responses and reduces risk of toxicity, while polysulfone, although biocompatible, may have limited sterilization compatibility and less optimal mechanical properties under physiological conditions. The enhanced safety profile of PEEK in medical applications is supported by extensive FDA approvals for implants, highlighting its preferred status in critical biomedical devices such as spinal implants and dental prosthetics.
Mechanical Strength and Durability
Polyether ether ketone (PEEK) exhibits superior mechanical strength and durability compared to polysulfone (PSU), making it ideal for high-stress medical device applications. PEEK offers excellent resistance to fatigue, wear, and chemical degradation, sustaining performance in sterilization processes such as autoclaving and gamma radiation. Polysulfone, while biocompatible and moderately durable, has lower tensile strength and is more prone to hydrolytic degradation, limiting its use in demanding mechanical environments.
Sterilization Compatibility and Resistance
Polyether ether ketone (PEEK) offers superior sterilization compatibility, withstanding repeated autoclaving, gamma radiation, and ethylene oxide without significant degradation, making it ideal for medical devices requiring rigorous sterilization protocols. Polysulfone (PSU) also demonstrates good resistance to steam sterilization and ethylene oxide but is less resistant to gamma and e-beam radiation, limiting its applications in devices exposed to high-energy sterilization methods. PEEK's exceptional chemical resistance and thermal stability ensure long-term performance and biocompatibility under various sterilization cycles, whereas polysulfone is more susceptible to polymer chain scission and dimensional changes under aggressive sterilization conditions.
Thermal Stability and Performance Under Stress
Polyether ether ketone (PEEK) offers superior thermal stability with a continuous service temperature up to 250degC, outperforming polysulfone (PSU), which typically withstands temperatures up to 160degC. PEEK maintains mechanical integrity and resists deformation under prolonged thermal stress, making it ideal for high-performance medical devices requiring autoclave sterilization and repeated thermal cycling. Polysulfone exhibits good toughness and impact resistance but can experience thermal softening and dimensional changes at elevated temperatures, limiting its application in high-heat or long-term stress environments.
Processing and Manufacturing Considerations
Polyether ether ketone (PEEK) offers superior thermal stability and chemical resistance, enabling high-temperature sterilization processes essential for medical devices, while its semi-crystalline structure requires precise injection molding parameters to avoid defects. Polysulfone (PSU) presents easier processing with a wider melt temperature range and excellent dimensional stability, supporting complex geometries and tight tolerances via injection molding and extrusion. Manufacturing PEEK demands controlled cooling rates and specialized tooling to maintain mechanical integrity, whereas PSU benefits from faster cycle times and lower processing costs, making material selection dependent on device performance requirements and production scale.
Regulatory Approvals and Standards Compliance
Polyether ether ketone (PEEK) and Polysulfone (PSU) exhibit distinct regulatory approvals and standards compliance profiles for medical devices, with PEEK often holding FDA clearance for implantable applications due to its biocompatibility and chemical resistance. PEEK complies with ISO 10993 standards for biological evaluation of medical devices, while Polysulfone meets USP Class VI certification, making it suitable for sterilizable medical equipment but less common in implants. Regulatory agencies favor PEEK in high-performance applications requiring long-term durability and regulatory adherence, whereas Polysulfone's compliance supports usage in non-implantable, sterilizable components.
Cost Analysis: PEEK vs. Polysulfone
Polyether ether ketone (PEEK) generally incurs higher initial material costs compared to polysulfone due to its superior mechanical properties and chemical resistance, which are critical for high-performance medical device applications. Polysulfone offers a cost-effective alternative with adequate toughness and thermal stability, making it suitable for devices with less demanding requirements. When considering total cost of ownership, PEEK may provide greater value through enhanced durability and longer service life, potentially reducing maintenance and replacement expenses.
Applications and Case Studies in Medical Devices
Polyether ether ketone (PEEK) and polysulfone (PSU) are widely utilized in medical devices due to their biocompatibility and mechanical properties. PEEK offers superior chemical resistance and high-temperature stability, making it ideal for orthopedic implants, spinal cages, and dental devices, as demonstrated in multiple case studies highlighting its durability and performance in load-bearing applications. Polysulfone is favored for sterilizable components such as dialysis membranes and surgical instrument handles, with clinical evaluations showing its robustness under repeated autoclave cycles and excellent dimensional stability.
Infographic: Polyether ether ketone vs Polysulfone for Medical device
 azmater.com
azmater.com