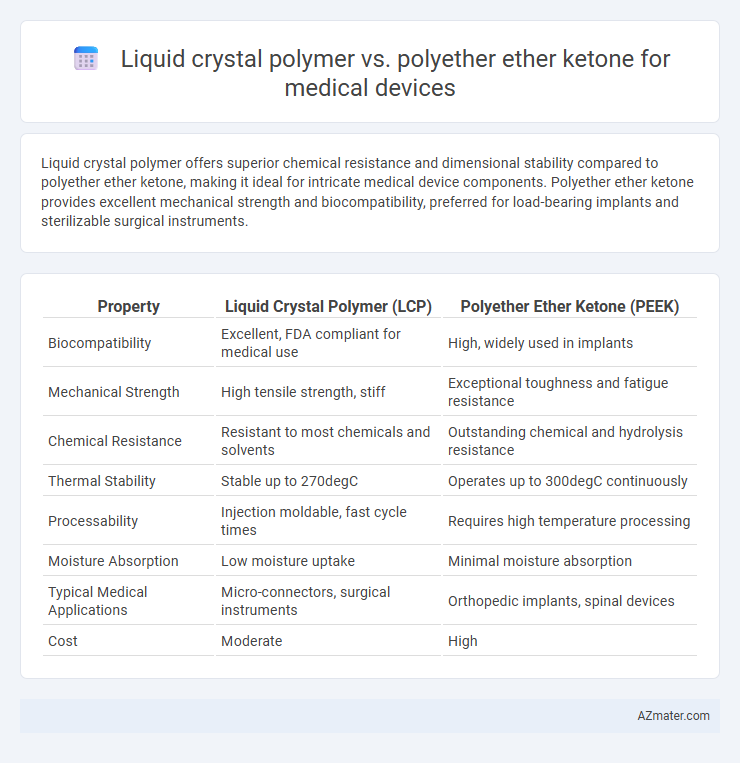
Liquid crystal polymer offers superior chemical resistance and dimensional stability compared to polyether ether ketone, making it ideal for intricate medical device components. Polyether ether ketone provides excellent mechanical strength and biocompatibility, preferred for load-bearing implants and sterilizable surgical instruments.
Table of Comparison
| Property | Liquid Crystal Polymer (LCP) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Biocompatibility | Excellent, FDA compliant for medical use | High, widely used in implants |
| Mechanical Strength | High tensile strength, stiff | Exceptional toughness and fatigue resistance |
| Chemical Resistance | Resistant to most chemicals and solvents | Outstanding chemical and hydrolysis resistance |
| Thermal Stability | Stable up to 270degC | Operates up to 300degC continuously |
| Processability | Injection moldable, fast cycle times | Requires high temperature processing |
| Moisture Absorption | Low moisture uptake | Minimal moisture absorption |
| Typical Medical Applications | Micro-connectors, surgical instruments | Orthopedic implants, spinal devices |
| Cost | Moderate | High |
Introduction to Liquid Crystal Polymer (LCP) and Polyether Ether Ketone (PEEK)
Liquid Crystal Polymer (LCP) and Polyether Ether Ketone (PEEK) are high-performance polymers widely used in medical device applications due to their exceptional mechanical strength, chemical resistance, and biocompatibility. LCP offers outstanding dimensional stability and excellent resistance to sterilization processes, making it suitable for precision components in minimally invasive surgical instruments. PEEK is favored for its superior fatigue resistance, high temperature tolerance, and radiolucency, often used in orthopedic implants and dental devices where long-term durability and compatibility with imaging are critical.
Material Composition and Structure Differences
Liquid crystal polymers (LCPs) possess a highly ordered molecular structure with rigid rod-like polymer chains that align to form liquid crystalline mesophases, offering superior chemical resistance and dimensional stability. Polyether ether ketone (PEEK) has a semi-crystalline structure composed of aromatic rings linked by ketone and ether groups, providing excellent mechanical strength and thermal stability. The distinctive molecular orientation of LCPs results in anisotropic properties beneficial for precision medical devices, while PEEK's isotropic structure ensures uniformity and durability in load-bearing implants.
Mechanical Properties Comparison
Liquid crystal polymer (LCP) offers exceptional tensile strength and dimensional stability, making it highly suitable for precision medical devices requiring durability and lightweight performance. Polyether ether ketone (PEEK) exhibits superior impact resistance and chemical resistance, providing excellent mechanical integrity under sterilization processes and bodily conditions. While LCP excels in stiffness and low thermal expansion, PEEK demonstrates better fatigue resistance and toughness, crucial for long-term implantable medical applications.
Chemical and Thermal Resistance
Liquid crystal polymers (LCPs) exhibit superior chemical resistance against a wide range of solvents, acids, and bases, making them ideal for medical devices exposed to harsh sterilization processes. Polyether ether ketone (PEEK) offers exceptional thermal stability with a continuous service temperature up to 250degC, outperforming LCPs in prolonged high-temperature environments. Both materials resist hydrolysis and biodegradation, but PEEK's enhanced mechanical strength and thermal endurance provide a reliable choice for implants and surgical instruments requiring sustained chemical and thermal resilience.
Biocompatibility and FDA Approvals
Liquid crystal polymer (LCP) exhibits superior biocompatibility with low cytotoxicity and excellent chemical resistance, making it suitable for implantable medical devices. Polyether ether ketone (PEEK) is widely FDA-approved for long-term use in spinal implants and orthopedic devices due to its proven biocompatibility and mechanical stability. Both materials meet stringent FDA regulations, but PEEK has broader approval history while LCP is gaining traction in microelectronic medical components.
Sterilization Methods and Performance
Liquid crystal polymer (LCP) and polyether ether ketone (PEEK) each exhibit distinct resistance to sterilization methods crucial for medical device applications. LCP shows excellent stability under gamma radiation and ethylene oxide sterilization but may degrade under prolonged steam autoclaving, whereas PEEK withstands steam sterilization, gamma radiation, and ethylene oxide with minimal impact on mechanical properties. PEEK's higher thermal resistance and chemical inertness offer superior performance in sterilization cycles common in medical device manufacturing, maintaining structural integrity and biocompatibility.
Processing and Manufacturing Considerations
Liquid crystal polymer (LCP) offers superior dimensional stability and excellent chemical resistance, enabling high-precision molding and minimal warpage for complex medical device components. Polyether ether ketone (PEEK) provides outstanding thermal stability and machinability, supporting sterilization processes but requiring higher processing temperatures and specialized equipment compared to LCP. Both materials demand strict control over drying and molding parameters to ensure biocompatibility and mechanical integrity in medical device manufacturing.
Common Medical Device Applications
Liquid crystal polymer (LCP) is widely used in medical devices requiring high chemical resistance and dimensional stability, such as catheter components and connectors, due to its excellent biocompatibility and low moisture absorption. Polyether ether ketone (PEEK) is favored for orthopedic implants, dental devices, and spinal cages because of its superior mechanical strength, radiolucency, and compatibility with sterilization processes. Both materials support complex designs and long-term implant performance, with LCP excelling in electronic medical parts and PEEK in structural medical applications.
Cost Analysis and Scalability
Liquid crystal polymer (LCP) offers cost advantages in medical device manufacturing due to its lower raw material cost and faster molding cycles compared to polyether ether ketone (PEEK), which commands higher prices due to superior thermal and chemical resistance. Scalability of LCP is enhanced by its compatibility with standard injection molding processes, enabling higher throughput and lower production costs at scale, whereas PEEK's high melting point necessitates specialized equipment and longer cycle times, limiting large-scale cost efficiency. Decision-makers prioritize LCP when balancing cost-sensitive applications requiring moderate performance, while PEEK is reserved for high-performance devices where material properties justify the premium.
Conclusion: Selecting the Ideal Polymer for Medical Devices
Liquid crystal polymer (LCP) offers exceptional chemical resistance, dimensional stability, and biocompatibility, making it suitable for intricate medical device components requiring precision. Polyether ether ketone (PEEK) provides superior mechanical strength, high temperature resistance, and excellent sterilization compatibility, ideal for load-bearing implants and reusable surgical instruments. Selecting the ideal polymer depends on specific device requirements, with LCP favored for micro-sized parts and electrical applications, while PEEK excels in structural components demanding durability and repeated sterilization.
Infographic: Liquid crystal polymer vs Polyether ether ketone for Medical device
 azmater.com
azmater.com