Fluoropolymers offer superior chemical resistance and biocompatibility for medical implants, while Polyetheretherketone (PEEK) provides exceptional mechanical strength and wear resistance. Choosing between fluoropolymer and PEEK depends on the implant's specific requirements for flexibility, durability, and tissue interaction.
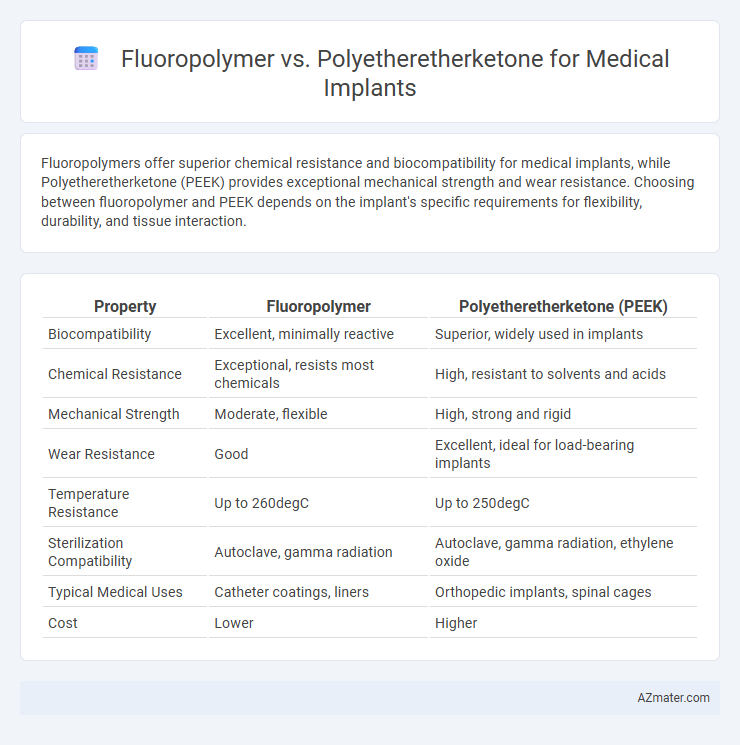
Table of Comparison
| Property | Fluoropolymer | Polyetheretherketone (PEEK) |
|---|---|---|
| Biocompatibility | Excellent, minimally reactive | Superior, widely used in implants |
| Chemical Resistance | Exceptional, resists most chemicals | High, resistant to solvents and acids |
| Mechanical Strength | Moderate, flexible | High, strong and rigid |
| Wear Resistance | Good | Excellent, ideal for load-bearing implants |
| Temperature Resistance | Up to 260degC | Up to 250degC |
| Sterilization Compatibility | Autoclave, gamma radiation | Autoclave, gamma radiation, ethylene oxide |
| Typical Medical Uses | Catheter coatings, liners | Orthopedic implants, spinal cages |
| Cost | Lower | Higher |
Introduction to Medical Implant Materials
Fluoropolymers and polyetheretherketone (PEEK) are critical materials in medical implant manufacturing due to their outstanding biocompatibility, chemical resistance, and mechanical properties. Fluoropolymers exhibit exceptional non-stick and low-friction characteristics, making them ideal for vascular and cardiovascular implants, whereas PEEK provides high strength, radiolucency, and thermal stability suitable for orthopedic and spinal implants. The choice between fluoropolymers and PEEK depends on specific implant requirements, including load-bearing capacity, sterilization methods, and long-term tissue interaction.
Overview of Fluoropolymers
Fluoropolymers, such as polytetrafluoroethylene (PTFE), are widely used in medical implants due to their excellent chemical resistance, low friction coefficient, and biocompatibility, making them suitable for applications like vascular grafts and catheter coatings. These materials exhibit high thermal stability and non-reactivity with bodily fluids, reducing the risk of inflammatory responses or rejection. In comparison to polyetheretherketone (PEEK), fluoropolymers offer superior lubricity and lower surface energy, which contribute to minimized tissue adhesion and improved implant longevity.
Overview of Polyetheretherketone (PEEK)
Polyetheretherketone (PEEK) is a high-performance thermoplastic widely used in medical implants due to its excellent biocompatibility, mechanical strength, and chemical resistance. Compared to fluoropolymers, PEEK offers superior structural integrity and radiolucency, making it ideal for load-bearing orthopedic devices and spinal implants. Its thermal stability and resistance to wear further enhance its durability in long-term implant applications.
Biocompatibility: Fluoropolymer vs PEEK
Fluoropolymers exhibit exceptional biocompatibility due to their chemical inertness, low surface energy, and resistance to protein adsorption, minimizing inflammatory responses in medical implants. Polyetheretherketone (PEEK) offers favorable biocompatibility with mechanical properties closely resembling human bone, promoting osseointegration and reducing implant rejection risks. Comparatively, fluoropolymers tend to be more chemically resistant, while PEEK provides superior structural strength and is widely preferred in load-bearing implant applications.
Mechanical Properties Comparison
Fluoropolymers exhibit excellent chemical resistance and low friction, but their mechanical properties, such as tensile strength and stiffness, are generally lower than those of polyetheretherketone (PEEK). PEEK offers superior mechanical strength, high tensile modulus, and excellent fatigue resistance, making it more suitable for load-bearing medical implants. The enhanced durability and biomechanical compatibility of PEEK ensure long-term performance and implant stability compared to fluoropolymers in demanding medical applications.
Chemical Resistance and Stability
Fluoropolymers exhibit exceptional chemical resistance to a wide range of aggressive solvents, acids, and bases, making them highly stable in harsh medical environments. Polyetheretherketone (PEEK) offers excellent mechanical strength and thermal stability alongside strong resistance to hydrolysis and oxidative degradation, ensuring long-term implant durability. Both materials provide biocompatibility, but fluoropolymers outperform PEEK in chemical inertness, while PEEK is superior in maintaining mechanical integrity under physiological stress.
Implant Longevity and Performance
Fluoropolymers offer exceptional chemical resistance and low friction, making them ideal for medical implants requiring minimal wear and high biocompatibility. Polyetheretherketone (PEEK) provides superior mechanical strength and excellent fatigue resistance, contributing to enhanced implant longevity and structural integrity under physiological loads. The choice between fluoropolymer and PEEK significantly impacts implant performance, with PEEK favored for load-bearing applications and fluoropolymers preferred for coatings and lubricant layers.
Manufacturing and Processing Considerations
Fluoropolymers offer excellent chemical resistance and low friction, making them suitable for medical implants requiring smooth surface finishes and biocompatibility, but they pose challenges during processing due to high melting points and sensitivity to thermal degradation. Polyetheretherketone (PEEK) provides superior mechanical strength and thermal stability with easier machining and molding capabilities, supporting complex implant geometries and sterilization methods such as autoclaving. Manufacturing fluoropolymer implants demands precise temperature control and specialty equipment to avoid material degradation, whereas PEEK's reliable thermal behavior allows for consistent manufacturing workflows and better scalability in medical device production.
Clinical Applications and Case Studies
Fluoropolymers exhibit exceptional chemical resistance and biocompatibility, making them ideal for vascular grafts and catheter coatings in medical implants, as evidenced by case studies highlighting reduced inflammation and thrombus formation. Polyetheretherketone (PEEK) is favored in orthopedic and spinal implants due to its superior mechanical strength, radiolucency, and osteointegration capabilities, validated by clinical trials demonstrating improved patient outcomes in spinal fusion surgeries. Comparative studies reveal fluoropolymers excel in soft tissue compatibility, while PEEK outperforms in load-bearing applications, guiding material selection based on specific clinical requirements.
Future Developments in Medical Implant Materials
Advancements in fluoropolymer materials for medical implants focus on enhancing biocompatibility, chemical inertness, and flexibility, meeting stringent FDA requirements for long-term use. Polyetheretherketone (PEEK) developments emphasize improved mechanical strength, radiolucency for better imaging compatibility, and surface modifications to promote osseointegration and reduce infection risks. Future medical implants will benefit from combining fluoropolymer chemical resistance with PEEK's structural integrity to create hybrid materials offering superior performance and patient outcomes.
Infographic: Fluoropolymer vs Polyetheretherketone for Medical Implant
 azmater.com
azmater.com