Zinc offers higher corrosion resistance and strength in alloys compared to tin, making it ideal for die-casting and galvanizing applications. Tin provides excellent solderability and non-toxicity, favored for coating steel to prevent rust and in electronics solder alloys.
Table of Comparison
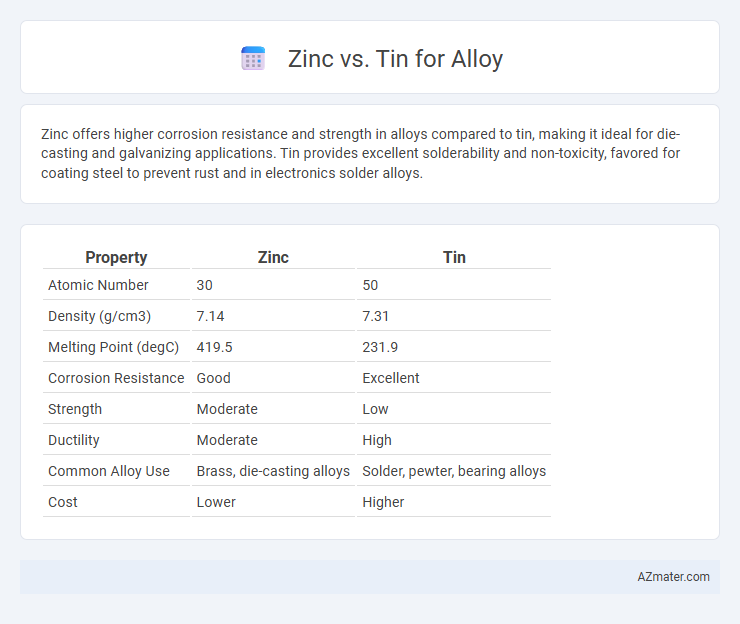
| Property | Zinc | Tin |
|---|---|---|
| Atomic Number | 30 | 50 |
| Density (g/cm3) | 7.14 | 7.31 |
| Melting Point (degC) | 419.5 | 231.9 |
| Corrosion Resistance | Good | Excellent |
| Strength | Moderate | Low |
| Ductility | Moderate | High |
| Common Alloy Use | Brass, die-casting alloys | Solder, pewter, bearing alloys |
| Cost | Lower | Higher |
Introduction to Zinc and Tin in Alloys
Zinc and tin are widely used metals in alloy formulations, each offering distinct properties that enhance metal performance. Zinc is primarily valued for its corrosion resistance and low melting point, making it essential in brass (copper-zinc) alloys and galvanized coatings. Tin, known for its excellent solderability and resistance to oxidation, plays a crucial role in bronze (copper-tin) alloys and lead-free solders, providing durability and improved mechanical strength.
Chemical and Physical Properties Comparison
Zinc exhibits a melting point of 419.5degC and density of 7.14 g/cm3, which contrasts with tin's lower melting point of 231.9degC and density of 7.31 g/cm3, influencing their alloy behavior and applications in corrosion resistance and malleability. Chemically, zinc is more reactive, forming protective oxide layers that enhance durability, whereas tin's inertness provides excellent solderability and resistance to oxidation. The choice between zinc and tin in alloys depends on desired characteristics such as tensile strength, ductility, and environmental resistance.
Historical Uses of Zinc and Tin in Alloy Making
Zinc has been historically utilized in alloy making primarily for brass production, where its corrosion resistance and malleability improved the durability and workability of the alloy in applications like coinage and musical instruments. Tin, known since ancient times, was a crucial component in bronze alloys, combining with copper to create high-strength, corrosion-resistant tools, weapons, and art objects essential to early civilizations. Both metals significantly influenced metallurgical advancements, with zinc enhancing modern brass compositions and tin's role in bronze shaping early technological development.
Common Alloys Containing Zinc and Tin
Common alloys containing zinc include brass, an alloy of copper and zinc known for its corrosion resistance and strength, and zinc-aluminum alloys valued for their durability and lightweight properties. Tin is a key component in bronze, an alloy of copper and tin, which is prized for its hardness and corrosion resistance, and in solder, an alloy of tin and lead or other metals used for joining metal parts. Both zinc and tin contribute essential properties to their respective alloys, enhancing mechanical performance and resistance to environmental factors.
Mechanical Strength: Zinc vs Tin Alloys
Zinc alloys typically offer higher mechanical strength and improved wear resistance compared to tin alloys, making them suitable for applications requiring durability and load-bearing capacity. Tin alloys, although softer and more malleable, excel in corrosion resistance and provide better ductility, which is beneficial for precision castings and electrical components. The choice between zinc and tin alloys depends on the specific mechanical strength requirements and environmental conditions of the application.
Corrosion Resistance Capabilities
Zinc offers superior corrosion resistance in alloy applications due to its ability to form a protective oxide layer that prevents further oxidation, making it ideal for galvanizing steel. Tin also provides good corrosion resistance, particularly against acidic environments, but is generally more susceptible to wear and corrosion over time compared to zinc. Alloys with higher zinc content typically exhibit enhanced durability and resistance to rust, especially in outdoor and marine environments.
Workability and Malleability Differences
Zinc offers excellent workability due to its moderate melting point and ability to be easily cast, stamped, and molded, making it ideal for die-casting alloys. Tin demonstrates superior malleability, allowing it to be shaped and bent without cracking, which is crucial for applications requiring fine detailing and thin sheets. While zinc alloys tend to be harder and more brittle, tin alloys provide greater flexibility and resistance to deformation, enhancing their suitability for use in coatings and soft soldering materials.
Cost and Availability of Zinc and Tin
Zinc is generally more cost-effective and widely available than tin, making it a preferred choice for alloy production where budget and supply stability are critical. Tin's higher market price and limited global reserves often lead to increased material costs and potential supply chain constraints. Industries favor zinc alloys for large-scale applications due to their economic advantages and abundant resource accessibility.
Environmental Impact and Sustainability
Zinc alloys exhibit lower toxicity and better recyclability compared to tin alloys, reducing environmental contamination and resource depletion. Tin mining often leads to significant habitat disruption and energy-intensive refining processes, whereas zinc can be recycled efficiently with less ecological footprint. Choosing zinc-based alloys supports sustainable manufacturing practices by minimizing hazardous waste and promoting circular economy principles.
Choosing the Right Metal for Your Alloy Applications
Zinc offers superior corrosion resistance and good ductility, making it ideal for die-casting and protective coatings in alloy applications. Tin provides excellent solderability and low toxicity, which benefits electronics and food-grade alloys. Selecting between zinc and tin depends on the alloy's mechanical requirements and environmental exposure to optimize performance and durability.
Infographic: Zinc vs Tin for Alloy
 azmater.com
azmater.com