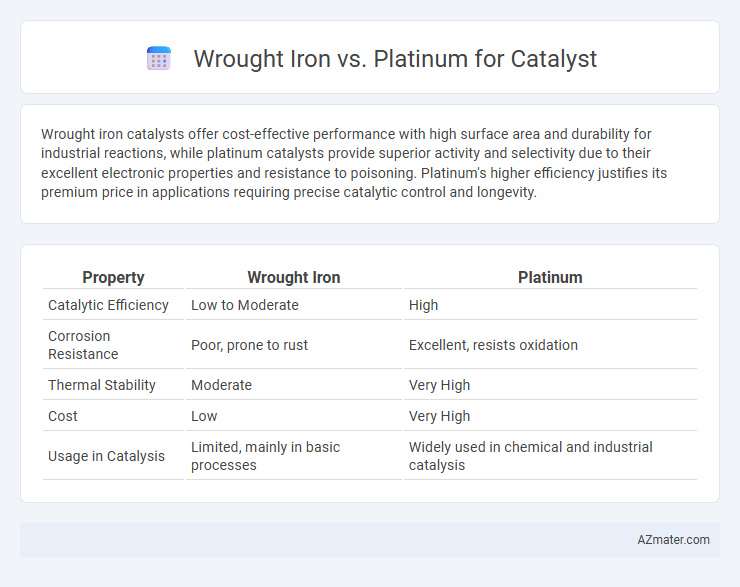
Wrought iron catalysts offer cost-effective performance with high surface area and durability for industrial reactions, while platinum catalysts provide superior activity and selectivity due to their excellent electronic properties and resistance to poisoning. Platinum's higher efficiency justifies its premium price in applications requiring precise catalytic control and longevity.
Table of Comparison
| Property | Wrought Iron | Platinum |
|---|---|---|
| Catalytic Efficiency | Low to Moderate | High |
| Corrosion Resistance | Poor, prone to rust | Excellent, resists oxidation |
| Thermal Stability | Moderate | Very High |
| Cost | Low | Very High |
| Usage in Catalysis | Limited, mainly in basic processes | Widely used in chemical and industrial catalysis |
Introduction to Catalysts: Wrought Iron and Platinum
Wrought iron and platinum serve as catalysts with distinct properties and applications in chemical reactions. Wrought iron is valued for its durability and cost-effectiveness, often used in industrial processes such as ammonia synthesis in the Haber-Bosch method. Platinum, characterized by its excellent catalytic activity and resistance to corrosion, is extensively employed in automotive catalytic converters and fine chemical production.
Chemical Properties Influencing Catalytic Performance
Wrought iron exhibits moderate catalytic activity due to its ability to facilitate electron transfer through variable oxidation states, primarily Fe2+ and Fe3+, enabling redox reactions in processes like Fischer-Tropsch synthesis. Platinum, characterized by its exceptional chemical stability and high electronegativity, offers superior catalytic performance by providing active sites for adsorption and activation of reactant molecules, particularly in hydrogenation and dehydrogenation reactions. The chemical inertness and resistance to oxidation of platinum result in prolonged catalyst life and higher turnover frequencies compared to wrought iron, which is more prone to surface oxidation and catalyst deactivation.
Efficiency Comparison: Wrought Iron vs Platinum
Platinum exhibits significantly higher catalytic efficiency than wrought iron due to its superior ability to adsorb and activate reactant molecules, leading to faster reaction rates and lower activation energy requirements. Wrought iron, while cheaper and more abundant, generally shows lower catalytic activity and durability, often requiring higher temperatures to achieve comparable catalytic performance. Platinum's enhanced electron density and surface properties enable more efficient catalytic cycles, particularly in hydrogenation and oxidation reactions, making it the preferred choice in industrial applications despite its higher cost.
Cost Analysis and Economic Viability
Wrought iron offers a significantly lower initial material cost compared to platinum, making it attractive for large-scale catalytic applications where budget constraints are critical. Platinum, despite its high expense, provides superior catalytic efficiency and durability, often resulting in longer operational life and reduced replacement frequency. Economic viability hinges on balancing the lower upfront cost of wrought iron against the higher performance and longevity of platinum catalysts, with total lifecycle cost analysis being essential for optimal decision-making.
Industrial Applications: Where Each Catalyst Excels
Wrought iron is widely utilized in industrial catalytic processes involving ammonia synthesis and Fischer-Tropsch reactions due to its excellent activity and cost-effectiveness in hydrogenation and nitrogen fixation. Platinum excels in high-value applications such as automotive catalytic converters and fuel cells, offering superior resistance to poisoning, high-temperature stability, and exceptional catalytic efficiency in oxidation and hydrogenation reactions. In industrial settings requiring durable, noble metal catalysts with precise control over reaction pathways, platinum's high catalytic turnover and selectivity outperform wrought iron despite the higher material cost.
Environmental Impact and Sustainability Factors
Wrought iron as a catalyst offers a lower environmental footprint due to its abundance, recyclability, and lower energy-intensive extraction compared to platinum, which requires mining rare and finite resources with significant ecological disruption. Platinum catalysts, while highly efficient and durable, pose sustainability challenges from limited availability and associated carbon emissions during extraction and refining. Iron-based catalysts contribute to greener chemical processes by enabling large-scale sustainable manufacturing and reducing reliance on scarce noble metals.
Durability and Lifespan of Wrought Iron vs Platinum Catalysts
Platinum catalysts exhibit superior durability and lifespan compared to wrought iron catalysts due to their high resistance to corrosion, oxidation, and thermal degradation. Wrought iron catalysts tend to degrade faster under harsh reaction conditions, leading to reduced catalytic efficiency and more frequent replacement. The longevity of platinum catalysts makes them more cost-effective over time in industrial applications despite their higher initial cost.
Reactivity and Selectivity in Key Chemical Reactions
Wrought iron exhibits moderate reactivity and selectivity in catalytic processes such as Fischer-Tropsch synthesis and ammonia synthesis, where its affordability and abundance make it a practical choice despite lower precision. Platinum outperforms wrought iron with superior catalytic activity and high selectivity, particularly in hydrogenation reactions and fuel cell applications, due to its unique electronic structure and resistance to poisoning. The trade-off between wrought iron's cost-effectiveness and platinum's enhanced performance is critical when optimizing catalysts for specific chemical reactions requiring tailored reactivity and selectivity profiles.
Availability and Resource Considerations
Wrought iron is abundant and widely accessible, making it a cost-effective catalyst option with minimal supply chain risks. Platinum, though highly efficient as a catalyst, is rare and expensive, leading to significant resource scarcity and elevated market prices. The choice between wrought iron and platinum hinges on balancing catalyst performance against availability and long-term resource sustainability.
Future Trends in Catalyst Development: Wrought Iron and Platinum
Future trends in catalyst development highlight the shift towards using wrought iron as a sustainable and cost-effective alternative to platinum, driven by increasing material scarcity and high prices of platinum. Advanced nanotechnology enhances wrought iron's catalytic activity and durability, making it competitive in industrial applications like hydrogen production and fuel cells. Research focuses on hybrid catalysts combining wrought iron's abundance with platinum's superior catalytic properties to optimize efficiency and reduce environmental impact.
Infographic: Wrought iron vs Platinum for Catalyst
 azmater.com
azmater.com