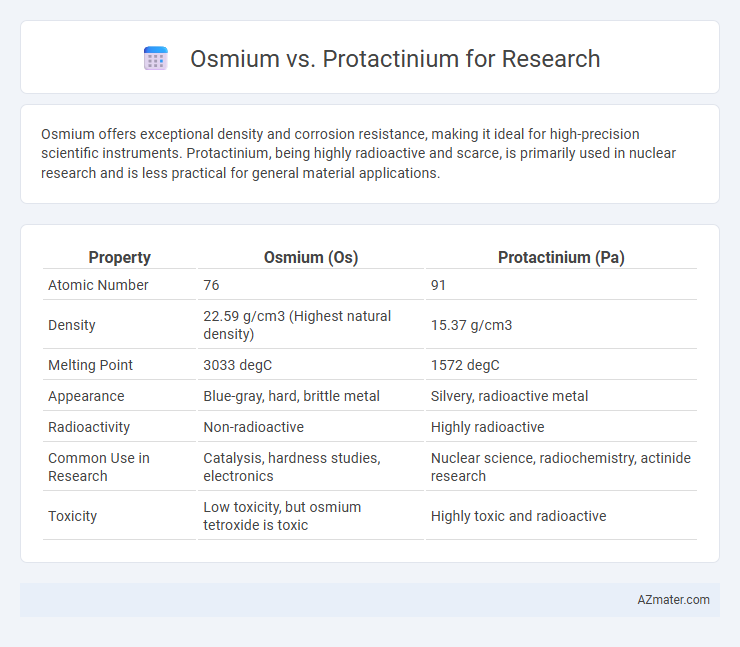
Osmium offers exceptional density and corrosion resistance, making it ideal for high-precision scientific instruments. Protactinium, being highly radioactive and scarce, is primarily used in nuclear research and is less practical for general material applications.
Table of Comparison
| Property | Osmium (Os) | Protactinium (Pa) |
|---|---|---|
| Atomic Number | 76 | 91 |
| Density | 22.59 g/cm3 (Highest natural density) | 15.37 g/cm3 |
| Melting Point | 3033 degC | 1572 degC |
| Appearance | Blue-gray, hard, brittle metal | Silvery, radioactive metal |
| Radioactivity | Non-radioactive | Highly radioactive |
| Common Use in Research | Catalysis, hardness studies, electronics | Nuclear science, radiochemistry, actinide research |
| Toxicity | Low toxicity, but osmium tetroxide is toxic | Highly toxic and radioactive |
Introduction to Osmium and Protactinium
Osmium, a dense transition metal with atomic number 76, is renowned for its exceptional hardness, high melting point, and applications in catalysis and materials science. Protactinium, element 91, is a rare actinide with significant radioactivity, primarily studied for its role in nuclear research and radioactive decay processes. Research involving osmium focuses on its chemical stability and catalytic properties, while protactinium studies prioritize nuclear properties and radiochemistry.
Elemental Properties Comparison
Osmium exhibits exceptional density at 22.59 g/cm3 and remarkable corrosion resistance, making it ideal for applications requiring durability and stability, while protactinium's radioactivity and scarcity limit its practical use in research but offer unique insights into nuclear chemistry and actinide behavior. Osmium's electron configuration [Xe] 4f14 5d6 6s2 provides high electron density and catalytic potential. Protactinium, with atomic number 91 and a half-life of 32,760 years for its most stable isotope Pa-231, is crucial for studying radioactive decay and nuclear transmutation processes.
Historical Discovery and Significance
Osmium, discovered in 1803 by Smithson Tennant, is notable for its extreme density and chemical inertness, making it essential in wear-resistant alloys and catalytic research. Protactinium, identified in 1917 by Kasimir Fajans and Oswald Helmuth Gohring, plays a crucial role in nuclear science due to its radioactive properties and position within the actinide series. Both elements have significantly advanced scientific understanding, with osmium aiding materials science and protactinium contributing to radiochemistry and nuclear physics.
Occurrence and Natural Abundance
Osmium occurs naturally in platinum ores with an average abundance of about 0.0016 parts per million in the Earth's crust, making it one of the rarest stable elements used in research. Protactinium is even rarer, with an estimated crustal abundance of only 0.0005 parts per million, primarily found in uranium ores as a decay product, which limits its availability for extensive scientific study. The scarcity and radioactivity of protactinium challenge its handling, while osmium's stable isotopes and higher natural abundance facilitate broader research applications.
Methods of Extraction and Purification
Osmium extraction primarily involves fractional crystallization from platinum ores and subsequent purification through high-temperature distillation of its volatile oxide, OsO4, allowing precise control over purity levels. Protactinium is extracted via complex multi-stage chemical procedures including solvent extraction and ion-exchange chromatography, designed to separate it from uranium and thorium decay products with high specificity. The distinct chemical properties of osmium and protactinium necessitate tailored purification methods, where osmium's volatility aids in refining, whereas protactinium requires careful handling of radioactive materials and multiple purification steps.
Physical and Chemical Behavior
Osmium exhibits extreme density and high corrosion resistance, making it valuable in studies of heavy metal alloys and catalytic processes involving oxidation reactions. Protactinium, characterized by its radioactive nature and complex electronic configuration, is essential for nuclear research and understanding actinide chemistry, particularly its oxidation states and reactivity with halogens. The contrasting physical stability of osmium versus protactinium's radioactivity underscores their distinctive roles in experimental and applied materials science.
Applications in Scientific Research
Osmium's exceptional density and stability make it ideal for high-precision scientific instruments and as a catalyst in chemical reactions, particularly in organic synthesis and ammonia production. Protactinium, though rarer and highly radioactive, is valuable primarily in nuclear research for studying actinide series behaviors and as a tracer in geochronology to date materials. The choice between osmium and protactinium depends on specific research needs, balancing osmium's chemical utility against protactinium's unique nuclear properties.
Safety, Handling, and Toxicity Concerns
Osmium, particularly osmium tetroxide, poses significant toxicity and requires stringent safety measures due to its volatile and highly toxic nature, necessitating proper ventilation and protective equipment during handling. Protactinium, while less commonly encountered, demands careful handling because of its radioactivity and chemical toxicity, with strict regulatory controls to mitigate radiation exposure and contamination risks. Both elements require specialized laboratory protocols, including containment and waste disposal procedures, ensuring researcher safety throughout experimental use.
Cost and Accessibility for Laboratories
Osmium, a rare and dense transition metal, is significantly more accessible for laboratories due to its availability as a byproduct of platinum mining, whereas protactinium is exceedingly scarce and primarily obtained from uranium decay, making it far more expensive and difficult to procure. The high cost of protactinium, driven by its radioactivity and limited supply, restricts its use to specialized nuclear research facilities with strict handling protocols, while osmium's relative affordability allows broader application in catalysis and material science research. Laboratories must weigh osmium's accessibility and manageable costs against protactinium's rarity and stringent regulatory requirements when selecting elements for experimental purposes.
Future Prospects and Research Opportunities
Osmium offers promising future prospects in catalytic applications and advanced material sciences due to its extreme density and chemical stability. Protactinium, though less explored, presents significant research opportunities in nuclear science and radiometric dating because of its unique radioactive properties and scarce natural availability. Investigating these elements can lead to breakthroughs in energy technologies and environmental monitoring through improved isotope utilization and novel compound synthesis.
Infographic: Osmium vs Protactinium for Research
 azmater.com
azmater.com