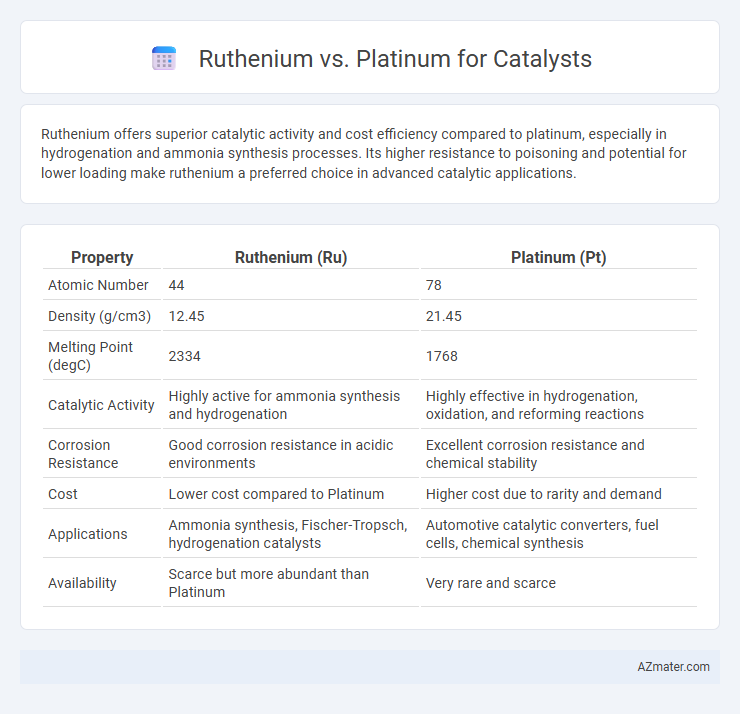
Ruthenium offers superior catalytic activity and cost efficiency compared to platinum, especially in hydrogenation and ammonia synthesis processes. Its higher resistance to poisoning and potential for lower loading make ruthenium a preferred choice in advanced catalytic applications.
Table of Comparison
| Property | Ruthenium (Ru) | Platinum (Pt) |
|---|---|---|
| Atomic Number | 44 | 78 |
| Density (g/cm3) | 12.45 | 21.45 |
| Melting Point (degC) | 2334 | 1768 |
| Catalytic Activity | Highly active for ammonia synthesis and hydrogenation | Highly effective in hydrogenation, oxidation, and reforming reactions |
| Corrosion Resistance | Good corrosion resistance in acidic environments | Excellent corrosion resistance and chemical stability |
| Cost | Lower cost compared to Platinum | Higher cost due to rarity and demand |
| Applications | Ammonia synthesis, Fischer-Tropsch, hydrogenation catalysts | Automotive catalytic converters, fuel cells, chemical synthesis |
| Availability | Scarce but more abundant than Platinum | Very rare and scarce |
Introduction to Ruthenium and Platinum Catalysts
Ruthenium and platinum are widely used transition metals in catalyst applications due to their exceptional chemical stability and catalytic efficiency. Ruthenium catalysts exhibit high activity in hydrogenation and ammonia synthesis reactions, benefiting from their unique electronic structure and ability to facilitate multi-electron transfer processes. Platinum catalysts are renowned for their superior performance in oxidation reactions and fuel cell technologies, driven by their excellent resistance to poisoning and high surface reactivity.
Chemical Properties and Reactivity of Ruthenium
Ruthenium exhibits exceptional catalytic properties due to its ability to adopt multiple oxidation states, enhancing its versatility in redox reactions compared to platinum. Ruthenium's higher catalytic activity in hydrogenation and ammonia synthesis arises from its strong adsorption capacity and unique electron configuration, which facilitates bond activation. Its resistance to poisoning and stability under harsh reaction conditions often make ruthenium a preferred choice over platinum in specific catalytic applications such as Fischer-Tropsch synthesis and oxidation reactions.
Chemical Properties and Reactivity of Platinum
Platinum exhibits remarkable chemical stability and resistance to oxidation, making it highly effective as a catalyst in various chemical reactions, including hydrogenation and oxidation processes. Its superior catalytic activity stems from the d-band electron configuration, which facilitates strong adsorption of reactants and intermediates, enhancing reaction rates. Unlike ruthenium, platinum is less prone to forming oxides under typical reaction conditions, thereby ensuring consistent catalytic performance and longevity.
Catalytic Efficiency: Ruthenium vs Platinum
Ruthenium exhibits higher catalytic efficiency than platinum in hydrogenation reactions due to its superior adsorption properties and electronic structure. Platinum, while effective across a broader range of reactions, often requires higher temperatures or pressures to achieve comparable activity levels. Ruthenium's enhanced catalytic performance makes it preferable in processes such as ammonia synthesis and selective hydrogenation.
Applications in Industrial Catalysis
Ruthenium exhibits exceptional activity in ammonia synthesis and Fischer-Tropsch processes, making it a preferred catalyst in hydrogenation and carbon monoxide conversion reactions. Platinum is widely favored for catalytic converters in automotive exhausts and in fuel cell technology due to its superior oxidation and reduction capabilities. Industrial catalysis benefits from ruthenium's cost-effectiveness and selectivity, whereas platinum's stability and resistance to poisoning ensure durability in harsh chemical environments.
Cost and Availability Comparison
Ruthenium offers a cost advantage over platinum due to its lower market price, often costing less than half per ounce, making it a more economical choice for catalytic applications. Platinum is more abundant in specialized extraction zones like South Africa but remains scarce globally, while ruthenium, primarily obtained as a byproduct of platinum mining in Russia and South Africa, benefits from slightly better overall availability. The combination of ruthenium's relative affordability and sufficient supply supports its growing adoption in catalytic converters and industrial catalysts compared to the traditionally used, but pricier, platinum.
Environmental Impact and Sustainability
Ruthenium catalysts demonstrate lower environmental impact due to their higher catalytic efficiency and ability to operate effectively at lower temperatures, reducing energy consumption and greenhouse gas emissions compared to platinum catalysts. Ruthenium's greater abundance relative to platinum contributes to improved sustainability by minimizing resource depletion and lowering extraction-related environmental damage. Despite platinum's established use in catalytic converters, ruthenium offers a more eco-friendly alternative by balancing performance with reduced environmental footprint and enhanced material availability.
Stability and Durability in Catalytic Processes
Ruthenium exhibits superior stability and durability in catalytic processes, particularly in harsh reaction environments such as ammonia synthesis and hydrogenation, due to its strong resistance to sintering and poisoning. Platinum, while highly active, often suffers from quicker deactivation caused by carbonaceous deposits and sulfur poisoning, reducing its long-term catalytic efficiency. Advanced Ruthenium catalysts demonstrate enhanced lifespan and maintained catalytic performance under extreme conditions compared to Platinum-based alternatives.
Recent Advances and Research Trends
Recent advances in Ruthenium-based catalysts emphasize their superior hydrogen evolution reaction (HER) performance and cost-effectiveness compared to Platinum, driven by enhanced nanostructuring techniques and surface engineering. Research trends highlight Ruthenium's increased durability and selectivity in ammonia synthesis and CO2 reduction applications, leveraging alloying with transition metals to optimize catalytic efficiency. Platinum remains the benchmark for oxygen reduction reactions (ORR), but efforts to reduce its usage focus on Ruthenium-platinum bimetallic catalysts designed to balance activity and material cost.
Conclusion: Choosing the Right Catalyst
Ruthenium offers higher catalytic activity and cost efficiency compared to platinum, making it ideal for applications requiring enhanced performance at a lower price point. Platinum provides superior stability and durability under harsh reaction conditions, which is crucial for long-term industrial processes. Selecting the right catalyst depends on balancing Ruthenium's economic advantages with Platinum's robustness to meet specific reaction requirements.
Infographic: Ruthenium vs Platinum for Catalyst
 azmater.com
azmater.com