Ruthenium offers superior deoxidizing properties compared to calcium due to its higher chemical stability and lower reactivity, enhancing metal purity and reducing oxidation risks. Calcium, while cost-effective and faster acting, can introduce impurities and less consistent deoxidation in high-temperature metallurgical processes.
Table of Comparison
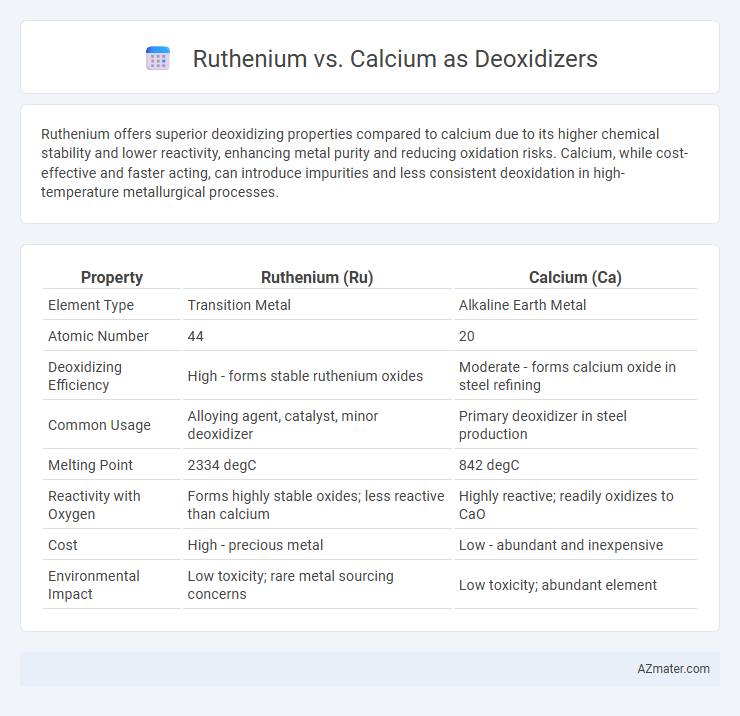
| Property | Ruthenium (Ru) | Calcium (Ca) |
|---|---|---|
| Element Type | Transition Metal | Alkaline Earth Metal |
| Atomic Number | 44 | 20 |
| Deoxidizing Efficiency | High - forms stable ruthenium oxides | Moderate - forms calcium oxide in steel refining |
| Common Usage | Alloying agent, catalyst, minor deoxidizer | Primary deoxidizer in steel production |
| Melting Point | 2334 degC | 842 degC |
| Reactivity with Oxygen | Forms highly stable oxides; less reactive than calcium | Highly reactive; readily oxidizes to CaO |
| Cost | High - precious metal | Low - abundant and inexpensive |
| Environmental Impact | Low toxicity; rare metal sourcing concerns | Low toxicity; abundant element |
Introduction: The Role of Deoxidizers in Metallurgy
Deoxidizers such as ruthenium and calcium play crucial roles in metallurgy by removing oxygen from molten metals to prevent oxidation defects and improve material properties. Ruthenium, a noble metal, acts as an effective trace deoxidizer enhancing corrosion resistance and mechanical strength, particularly in specialized alloys. Calcium, in contrast, is widely used for its high oxygen affinity and cost-effectiveness, facilitating cleaner steel production by forming stable calcium oxides that are easily removed during refining.
Overview of Ruthenium as a Deoxidizer
Ruthenium, a platinum-group metal, is highly effective as a deoxidizer due to its strong affinity for oxygen, forming stable ruthenium oxides that improve metal purity and reduce porosity. Compared to calcium, which is commonly used for its cost-effectiveness and ability to modify inclusion shapes, ruthenium offers superior oxidation resistance and enhances the mechanical properties of steel by minimizing oxide inclusions. Its catalytic properties also promote cleaner molten metal, making ruthenium a valuable additive in high-performance alloy production despite its higher cost.
Overview of Calcium as a Deoxidizer
Calcium is widely used as an effective deoxidizer in steelmaking due to its strong affinity for oxygen and sulfur, improving the cleanliness and mechanical properties of the final product. It forms stable oxides and sulfides, which float to the slag layer, thus reducing non-metallic inclusions and enhancing steel quality. Compared to ruthenium, calcium is more cost-effective and commonly employed in industrial applications for refining molten metals.
Chemical Properties: Ruthenium vs Calcium
Ruthenium exhibits superior catalytic activity and chemical stability compared to calcium, with a high melting point of 2334degC and resistance to oxidation, making it effective in deoxidation processes at elevated temperatures. Calcium, having a melting point of 842degC, is highly reactive with oxygen, rapidly forming calcium oxide, which efficiently removes oxygen impurities but lacks Ruthenium's catalytic versatility. The choice between Ruthenium and Calcium as deoxidizers depends on the desired reaction conditions, with Ruthenium favored in high-temperature, precision applications and Calcium preferred for cost-effective, rapid oxygen removal.
Efficiency in Oxygen Removal: Comparative Analysis
Ruthenium exhibits superior efficiency in oxygen removal compared to calcium due to its higher oxygen affinity and stability of ruthenium oxides, resulting in enhanced deoxidation performance in steelmaking. Calcium, though effective as a deoxidizer, forms less stable oxides and may require higher concentrations to achieve comparable oxygen reduction. The thermodynamic properties and reaction kinetics favor ruthenium, making it a more efficient choice for minimizing dissolved oxygen levels in metallurgical processes.
Impact on Metal Purity and Quality
Ruthenium, as a deoxidizer, significantly enhances metal purity by effectively reducing oxygen content with minimal slag formation, resulting in superior metal quality and improved mechanical properties. Calcium, while also used as a deoxidizer, may introduce inclusions or form calcium aluminate phases that can affect the final metal cleanliness and quality. The choice between ruthenium and calcium directly influences the efficiency of oxygen removal and the metallurgical characteristics of the finished product.
Cost Comparison: Ruthenium vs Calcium
Ruthenium, a rare and precious metal, exhibits significantly higher costs compared to calcium, which is abundant and inexpensive as a deoxidizer in metallurgical processes. The market price of ruthenium often exceeds several hundred dollars per gram, whereas calcium compounds are priced orders of magnitude lower, making calcium a cost-effective choice for large-scale industrial applications. Despite ruthenium's superior catalytic properties, its expense limits widespread use, especially when economic efficiency is paramount.
Environmental and Safety Considerations
Ruthenium, as a deoxidizer, presents fewer environmental hazards due to its stable chemical nature and reduced toxicity compared to calcium, which can produce corrosive and reactive byproducts, increasing risks during handling and disposal. Calcium's high reactivity with water releases hydrogen gas, posing explosion risks and requiring stringent safety protocols, while ruthenium's inertness minimizes such hazards. The lower environmental impact and safer operational profile make ruthenium a preferred choice for deoxidation in sensitive industrial processes focused on sustainability and worker safety.
Industrial Applications and Suitability
Ruthenium and calcium serve distinct roles as deoxidizers in industrial metallurgy, with ruthenium often preferred for its superior ability to enhance alloy strength and corrosion resistance, particularly in high-performance stainless steels and superalloys. Calcium excels in heavy steel manufacturing due to its efficiency in removing sulfur and oxygen impurities, improving ductility and weldability in structural steel and cast iron. The choice between ruthenium and calcium depends on the specific industrial application requirements, cost considerations, and desired metallurgical properties.
Conclusion: Selecting the Optimal Deoxidizer
Ruthenium, a rare transition metal, offers superior deoxidation efficiency and enhanced corrosion resistance in steelmaking compared to calcium, which is more cost-effective but less effective in removing oxygen. Optimal deoxidizer selection depends on specific production goals, where ruthenium excels in high-performance alloys requiring precise oxygen control, while calcium suits general steel refining with budget constraints. Balancing cost, deoxidation capability, and application requirements is essential for choosing the most suitable deoxidizer in metallurgical processes.
Infographic: Ruthenium vs Calcium for Deoxidizer
 azmater.com
azmater.com