Rhodium offers superior corrosion resistance, hardness, and a highly reflective finish compared to zinc, making it ideal for high-end electroplating applications. Zinc provides cost-effective protection against rust and corrosion, commonly used for industrial and automotive parts with moderate durability requirements.
Table of Comparison
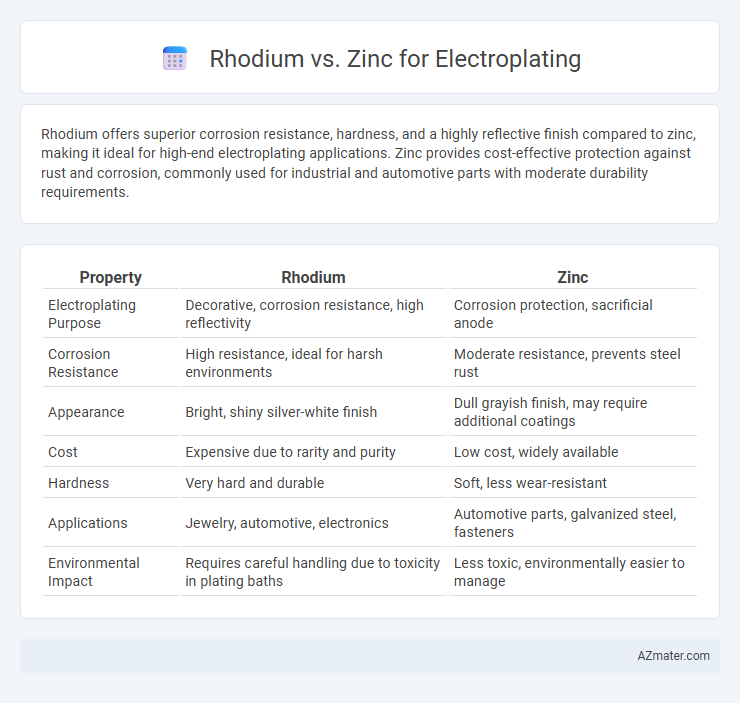
| Property | Rhodium | Zinc |
|---|---|---|
| Electroplating Purpose | Decorative, corrosion resistance, high reflectivity | Corrosion protection, sacrificial anode |
| Corrosion Resistance | High resistance, ideal for harsh environments | Moderate resistance, prevents steel rust |
| Appearance | Bright, shiny silver-white finish | Dull grayish finish, may require additional coatings |
| Cost | Expensive due to rarity and purity | Low cost, widely available |
| Hardness | Very hard and durable | Soft, less wear-resistant |
| Applications | Jewelry, automotive, electronics | Automotive parts, galvanized steel, fasteners |
| Environmental Impact | Requires careful handling due to toxicity in plating baths | Less toxic, environmentally easier to manage |
Introduction to Electroplating
Electroplating involves depositing a thin metal coating onto a substrate using an electric current, enhancing corrosion resistance and aesthetic appeal. Rhodium is prized for its superior hardness, reflectivity, and corrosion resistance, making it ideal for high-end jewelry and electronics. Zinc, valued for cost-effectiveness and sacrificial corrosion protection, is commonly used in automotive and construction industries to prevent rust.
Overview of Rhodium and Zinc
Rhodium, a rare and precious metal belonging to the platinum group, offers exceptional corrosion resistance, high reflectivity, and superior hardness, making it ideal for decorative and protective electroplating applications. Zinc, an abundant and cost-effective metal, provides excellent sacrificial protection against rust and corrosion, widely used for coating steel to enhance durability. Electroplating with rhodium ensures a bright, highly reflective finish with outstanding wear resistance, while zinc plating prioritizes affordability and corrosion prevention for industrial purposes.
Key Properties of Rhodium in Electroplating
Rhodium exhibits exceptional corrosion resistance, high reflectivity, and superior hardness, making it ideal for electroplating applications requiring durability and a brilliant finish. Its chemical inertness ensures long-lasting protection against tarnish and oxidation, especially in jewelry and automotive parts. The metal's excellent conductivity and ability to form a thin, uniform coating also enhance performance and aesthetic appeal in various industrial uses.
Key Properties of Zinc in Electroplating
Zinc is widely favored in electroplating for its excellent corrosion resistance and ability to provide a uniform, protective layer on steel and iron substrates. Its key properties include good adhesion, cost-effectiveness, and the formation of a sacrificial anode that prevents rust by corroding preferentially. The zinc layer also enhances paint adhesion and can be alloyed for improved mechanical strength and appearance.
Electroplating Techniques for Rhodium and Zinc
Rhodium electroplating employs a high-current density process with an acidic bath containing rhodium sulfate and sulfuric acid, producing a hard, corrosion-resistant, and highly reflective coating ideal for fine jewelry and electronics. Zinc electroplating uses a mildly alkaline or acidic cyanide-free solution with zinc sulfate or chloride, offering excellent corrosion protection especially for steel components through uniform deposition. Pulse plating and barrel plating techniques enhance coating uniformity and adhesion for both metals, with rhodium favoring rack plating for precision and zinc suited to mass production applications.
Corrosion Resistance: Rhodium vs Zinc
Rhodium offers superior corrosion resistance compared to zinc due to its inert, non-reactive surface that prevents oxidation and tarnishing in harsh environments. Zinc, while providing sacrificial protection through galvanic corrosion, tends to degrade over time when exposed to moisture and acidic conditions. The high durability and chemical stability of rhodium make it the preferred choice for long-lasting, corrosion-resistant electroplated finishes.
Cost Comparison: Rhodium vs Zinc Electroplating
Rhodium electroplating is significantly more expensive than zinc due to rhodium's rarity and high market price, often exceeding zinc costs by ten to twenty times per ounce. Zinc plating remains a cost-effective option for large-scale industrial applications because of its abundant availability and lower material price. However, the durability and corrosion resistance of rhodium can justify its premium cost in specialized uses such as jewelry and high-end electronics.
Common Applications of Rhodium and Zinc Plating
Rhodium plating is commonly used in jewelry, automotive parts, and electrical contacts due to its superior corrosion resistance, high reflectivity, and excellent hardness. Zinc plating finds widespread application in protecting steel fasteners, automotive components, and industrial machinery from rust and environmental degradation. Both metals enhance durability and appearance but cater to different industry-specific requirements based on their unique electrochemical properties.
Environmental and Safety Considerations
Rhodium electroplating offers superior corrosion resistance and a non-toxic finish, making it environmentally favorable compared to zinc, which can produce hazardous waste containing heavy metals and requires stringent disposal measures. Zinc plating often involves the use of cyanide baths, posing significant safety risks to workers due to toxic exposure, whereas rhodium plating typically uses safer, non-cyanide solutions. The environmental impact of zinc includes potential soil and water contamination from plating effluents, while rhodium's stable and inert coating reduces environmental hazards during the product lifecycle.
How to Choose Between Rhodium and Zinc for Electroplating
Choosing between rhodium and zinc for electroplating depends on factors like corrosion resistance, appearance, and application requirements. Rhodium offers superior corrosion resistance, a bright white finish, and is ideal for high-end jewelry or automotive parts with demanding environmental exposure. Zinc provides cost-effective corrosion protection with a duller finish, suitable for industrial components requiring budget-friendly, functional coatings rather than decorative appeal.
Infographic: Rhodium vs Zinc for Electroplating
 azmater.com
azmater.com