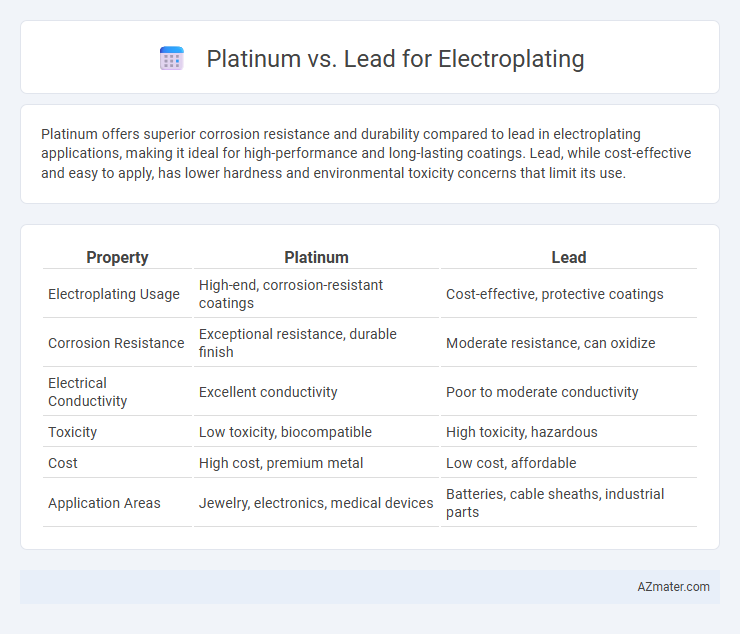
Platinum offers superior corrosion resistance and durability compared to lead in electroplating applications, making it ideal for high-performance and long-lasting coatings. Lead, while cost-effective and easy to apply, has lower hardness and environmental toxicity concerns that limit its use.
Table of Comparison
| Property | Platinum | Lead |
|---|---|---|
| Electroplating Usage | High-end, corrosion-resistant coatings | Cost-effective, protective coatings |
| Corrosion Resistance | Exceptional resistance, durable finish | Moderate resistance, can oxidize |
| Electrical Conductivity | Excellent conductivity | Poor to moderate conductivity |
| Toxicity | Low toxicity, biocompatible | High toxicity, hazardous |
| Cost | High cost, premium metal | Low cost, affordable |
| Application Areas | Jewelry, electronics, medical devices | Batteries, cable sheaths, industrial parts |
Introduction to Electroplating
Electroplating involves depositing a thin layer of metal onto a substrate through an electrolytic process, enhancing corrosion resistance and aesthetic appeal. Platinum offers superior conductivity, chemical stability, and resistance to oxidation compared to lead, making it ideal for high-performance applications. Lead is more affordable but prone to corrosion and environmental concerns, limiting its use in precision electroplating processes.
Overview of Platinum and Lead as Electroplating Materials
Platinum offers excellent corrosion resistance, high conductivity, and superior durability, making it ideal for high-performance electroplating applications such as jewelry and electronics. Lead, while less conductive and more prone to corrosion, is valued for its cost-effectiveness and ease of plating in industrial applications requiring thick, protective coatings. The choice between platinum and lead depends on required conductivity, corrosion resistance, cost constraints, and environmental regulations.
Chemical Properties: Platinum vs Lead
Platinum exhibits exceptional chemical stability, resisting oxidation and corrosion due to its noble metal status, making it ideal for high-durability electroplating. Lead, in contrast, is more reactive and prone to oxidation and corrosion, which limits its long-term performance in plating applications. The superior chemical inertness of platinum ensures a non-tarnishing, highly conductive coating compared to the less durable and more toxic lead.
Electroplating Efficiency and Performance Comparison
Platinum exhibits superior electroplating efficiency due to its excellent electrical conductivity and chemical stability, resulting in uniform, durable coatings with minimal defects. Lead, while cost-effective, demonstrates lower performance with increased porosity and reduced corrosion resistance in plated layers. The enhanced catalytic properties of platinum significantly improve deposition rates and coating adhesion, making it the preferred choice for high-precision electroplating applications.
Corrosion Resistance: Platinum vs Lead
Platinum exhibits superior corrosion resistance compared to lead, making it highly effective for electroplating applications in harsh chemical environments. Its inert nature prevents oxidation and degradation, ensuring long-lasting protective coatings. Lead, while resistant to some acids, is more prone to corrosion and wear, which limits its durability in electroplating processes.
Cost Analysis: Platinum Versus Lead
Platinum electroplating commands a significantly higher cost due to the precious metal's rarity and superior corrosion resistance, while lead offers a low-cost alternative with adequate conductivity but limited durability and environmental concerns. The price per ounce of platinum often exceeds $1,000, making it impractical for large-scale or budget-sensitive applications compared to lead, which costs only a few dollars per pound. Evaluating the total cost must include not only the raw material expense but also operational factors such as plating bath life, waste treatment, and regulatory compliance linked to lead toxicity.
Environmental and Safety Considerations
Platinum electroplating poses fewer environmental hazards compared to lead, as platinum is non-toxic and stable, minimizing harmful chemical runoff and reducing ecological impact. Lead electroplating releases toxic lead compounds, posing significant health risks such as lead poisoning and environmental contamination of soil and water. Strict safety protocols and waste management practices are essential when using lead, while platinum plating processes require less stringent controls due to its inert nature.
Industrial Applications of Platinum and Lead Electroplating
Platinum electroplating offers superior corrosion resistance, excellent wear properties, and high conductivity, making it ideal for industrial applications such as automotive components, electronic connectors, and laboratory equipment. Lead electroplating provides cost-effective protection against corrosion and electrical conductivity in applications like battery grids, cable sheathing, and radiation shielding. The choice between platinum and lead electroplating depends on the required durability, cost considerations, and specific environmental conditions in industrial settings.
Lifespan and Maintenance Requirements
Platinum offers superior lifespan in electroplating due to its excellent corrosion resistance and durability, reducing the need for frequent re-coating compared to lead, which tends to degrade faster under electrochemical conditions. Maintenance requirements for platinum-plated components are minimal, as the metal's inertness prevents oxidation and surface wear, whereas lead-plated surfaces require regular inspections and potential reapplication to maintain conductivity and protect against corrosion. The high initial cost of platinum is offset by its extended service life and reduced maintenance frequency, making it a cost-effective choice for long-term electroplating applications.
Conclusion: Choosing Between Platinum and Lead for Electroplating
Platinum offers superior corrosion resistance, conductivity, and long-term durability for electroplating applications, making it ideal for high-performance and precision uses despite its higher cost. Lead, while more affordable and easier to work with, provides adequate protection and conductivity for less demanding industrial coatings but lacks the longevity and environmental safety of platinum. Selecting between platinum and lead depends on budget constraints, desired coating quality, and specific application requirements in electroplating processes.
Infographic: Platinum vs Lead for Electroplating
 azmater.com
azmater.com