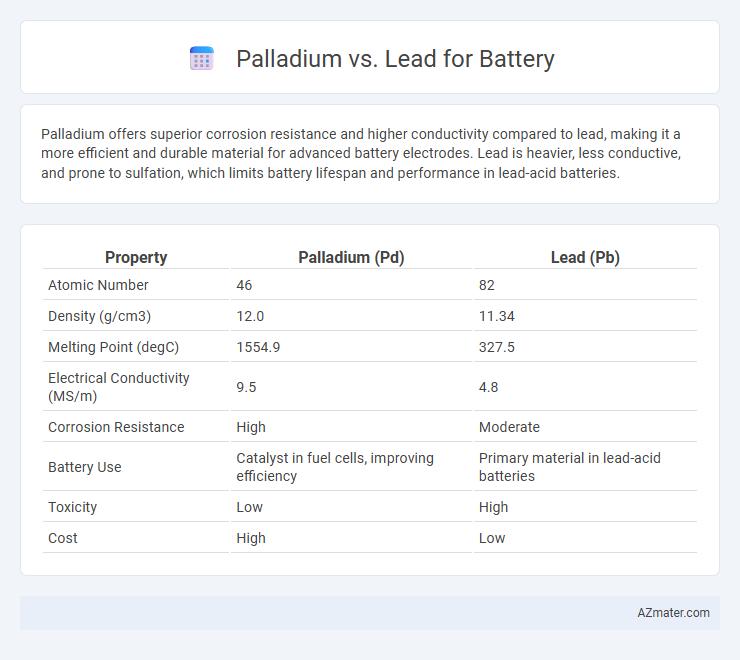
Palladium offers superior corrosion resistance and higher conductivity compared to lead, making it a more efficient and durable material for advanced battery electrodes. Lead is heavier, less conductive, and prone to sulfation, which limits battery lifespan and performance in lead-acid batteries.
Table of Comparison
| Property | Palladium (Pd) | Lead (Pb) |
|---|---|---|
| Atomic Number | 46 | 82 |
| Density (g/cm3) | 12.0 | 11.34 |
| Melting Point (degC) | 1554.9 | 327.5 |
| Electrical Conductivity (MS/m) | 9.5 | 4.8 |
| Corrosion Resistance | High | Moderate |
| Battery Use | Catalyst in fuel cells, improving efficiency | Primary material in lead-acid batteries |
| Toxicity | Low | High |
| Cost | High | Low |
Introduction to Palladium and Lead in Batteries
Palladium and lead serve distinct roles in battery technology, with lead predominantly used in lead-acid batteries due to its cost-effectiveness and reliable electrochemical properties. Palladium, a precious metal with exceptional catalytic activity, is less common but increasingly explored for enhancing battery performance in advanced energy storage systems. Comparing these elements highlights lead's dominance in traditional automotive batteries and palladium's potential in high-efficiency, next-generation electrodes.
Chemical Properties: Palladium vs Lead
Palladium exhibits excellent catalytic properties and high corrosion resistance, making it ideal for enhancing battery efficiency and longevity, whereas lead is prized for its high electrical conductivity and stability in acidic environments typical of lead-acid batteries. Palladium's ability to absorb hydrogen facilitates improved electrode reactions, while lead's dense atomic structure supports energy storage through reversible electrochemical reactions. The chemical inertness of palladium contrasts with lead's tendency to form lead sulfate during discharge, influencing the overall battery cycle performance and lifespan.
Energy Density Comparison
Palladium-based batteries typically offer higher energy density compared to lead-acid batteries, enabling longer runtime and more efficient energy storage in compact sizes. Lead-acid batteries usually have an energy density ranging between 30-50 Wh/kg, while palladium-enhanced technologies can reach significantly higher values, improving power-to-weight ratios. The superior energy density of palladium batteries supports advanced applications requiring lightweight and high-capacity energy solutions.
Environmental Impact and Sustainability
Palladium-based batteries offer improved energy efficiency and longer lifespan compared to traditional lead-acid batteries, resulting in reduced environmental waste and lower resource consumption. Lead-acid batteries pose significant environmental challenges due to lead toxicity and recycling difficulties, which can lead to soil and water contamination if improperly managed. Transitioning to palladium-enhanced battery technologies supports sustainability goals by minimizing hazardous material use and promoting eco-friendly energy storage solutions.
Cost Analysis of Palladium and Lead
Palladium batteries exhibit significantly higher costs compared to lead-acid batteries due to the scarcity and high market price of palladium, which currently trades around $2,000 per ounce, whereas lead remains abundant and priced under $2 per pound. The production expenses and raw material sourcing for palladium-based batteries contribute to their limited commercial adoption, making lead-acid batteries the more cost-effective choice for large-scale energy storage applications. Cost efficiency in lead batteries stems from their established supply chain, recyclability rates exceeding 90%, and lower material costs, offering superior affordability for industries prioritizing budget constraints.
Battery Lifespan and Performance
Palladium-based batteries exhibit significantly higher battery lifespan and enhanced performance compared to traditional lead-acid batteries, offering improved charge retention and faster energy discharge rates. The superior catalytic properties of palladium contribute to greater electrochemical stability and reduced degradation over repeated charge cycles. Lead batteries, while cost-effective and widely used, generally suffer from shorter cycle life and performance decline due to sulfation and corrosion issues.
Safety Concerns and Toxicity
Palladium-based batteries offer significantly lower toxicity compared to lead-acid batteries, reducing environmental and health hazards during manufacturing, use, and disposal. Lead batteries pose serious safety concerns due to lead's neurotoxic properties and the risk of acid leakage, which can cause chemical burns and environmental contamination. The use of palladium enhances battery safety by minimizing hazardous material exposure and improving overall ecological sustainability.
Market Availability and Resource Scarcity
Palladium is significantly scarcer than lead, with global reserves concentrated primarily in Russia and South Africa, limiting its market availability and driving high prices. Lead, abundant and widely distributed, maintains stable market availability due to extensive recycling in the battery industry. The scarcity of palladium challenges its large-scale adoption in batteries, whereas lead's abundance supports its continued dominance in lead-acid battery manufacturing.
Technological Innovations Using Palladium and Lead
Technological innovations in batteries using palladium focus on enhancing catalytic efficiency and durability, enabling faster charge cycles and improved energy density in fuel cells. Lead-based batteries benefit from advances in lead-acid chemistry, such as carbon-doping techniques and nanostructured lead components, which increase cycle life and reduce sulfation. Both metals contribute uniquely to evolving energy storage solutions, with palladium driving high-performance fuel cell development and lead advancing cost-effective, reliable stationary storage systems.
Future Prospects in Battery Technology
Palladium offers superior catalytic properties and corrosion resistance, positioning it as a promising material for next-generation batteries with enhanced performance and longevity. Lead, while traditionally dominant in lead-acid batteries due to low cost and recyclability, faces limitations in energy density and efficiency that restrict its future scalability. Emerging battery technologies increasingly favor palladium alloys and nanostructures to drive advancements in electric vehicle range and fast-charging capabilities, signaling a shift toward palladium-based solutions in the evolving energy storage market.
Infographic: Palladium vs Lead for Battery
 azmater.com
azmater.com