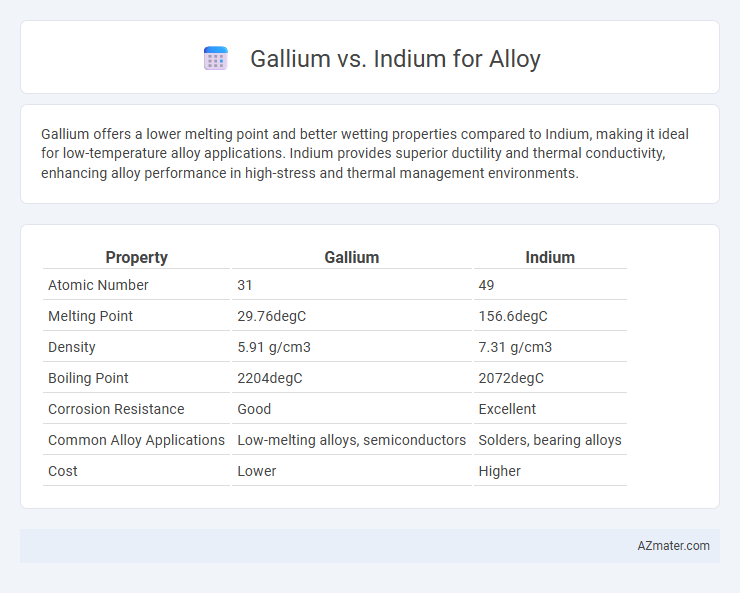
Gallium offers a lower melting point and better wetting properties compared to Indium, making it ideal for low-temperature alloy applications. Indium provides superior ductility and thermal conductivity, enhancing alloy performance in high-stress and thermal management environments.
Table of Comparison
| Property | Gallium | Indium |
|---|---|---|
| Atomic Number | 31 | 49 |
| Melting Point | 29.76degC | 156.6degC |
| Density | 5.91 g/cm3 | 7.31 g/cm3 |
| Boiling Point | 2204degC | 2072degC |
| Corrosion Resistance | Good | Excellent |
| Common Alloy Applications | Low-melting alloys, semiconductors | Solders, bearing alloys |
| Cost | Lower | Higher |
Introduction to Gallium and Indium Alloys
Gallium and indium alloys exhibit unique properties crucial for advanced electronics and thermal management applications. Gallium alloys, particularly those mixed with indium and tin, are favored for their low melting points and excellent wetting ability on various substrates, making them ideal for flexible electronics and thermal interface materials. Indium alloys enhance ductility and corrosion resistance, contributing to improved mechanical strength and reliability in soldering and bonding processes.
Physical Properties Comparison
Gallium exhibits a melting point of 29.76degC and a density of 5.91 g/cm3, while Indium's melting point is significantly higher at 156.6degC with a density of 7.31 g/cm3, making Indium more suitable for high-temperature alloy applications. Gallium's low melting point allows it to remain in liquid form near room temperature, enhancing its use in low melting alloys and thermal interface materials. Indium offers superior ductility and corrosion resistance, contributing to improved mechanical stability and longevity in alloy compositions.
Chemical Stability and Reactivity
Gallium exhibits greater chemical stability than indium, resisting oxidation and corrosion at room temperature, which makes it ideal for long-lasting alloys in electronic components. Indium, while softer and more reactive, forms alloys with lower melting points and better malleability but is prone to oxidation in air, requiring protective environments. The differing reactivities influence their alloy applications, with gallium preferred for stability and indium chosen for applications requiring enhanced ductility and solderability.
Melting Points and Alloy Formation
Gallium has a melting point of 29.76degC, making it a low-melting metal that easily forms alloys with metals like aluminum and indium. Indium, with a melting point of 156.6degC, provides higher thermal stability when alloyed, enabling the creation of materials suitable for high-temperature applications. The combination of gallium and indium in alloys allows tuning of melting points and enhances properties like malleability and corrosion resistance for electronics and thermal interface materials.
Applications in Electronics
Gallium and indium are critical elements in the electronics industry, particularly for alloy applications in semiconductors and conductive materials. Gallium-based alloys such as gallium arsenide (GaAs) are essential for high-speed integrated circuits, optoelectronic devices, and solar cells due to their superior electron mobility and direct bandgap properties. Indium, often alloyed in indium tin oxide (ITO), provides transparent conductive layers vital for touch screens, flat-panel displays, and photovoltaic cells, while its low melting point alloys are used in thermal interface materials for efficient heat dissipation in electronic devices.
Environmental and Health Considerations
Gallium and indium, both used in alloys such as low-melting point metals and semiconductors, differ significantly in environmental and health impacts. Gallium is relatively non-toxic and poses lower environmental risks due to its stable chemical behavior and minimal bioaccumulation, making it safer for widespread industrial use. Indium, while essential for technologies like LCD screens, can accumulate in the environment and potentially cause toxicity to aquatic life and humans upon prolonged exposure, necessitating careful handling and disposal protocols to mitigate adverse effects.
Cost and Availability
Gallium is more abundant and cost-effective compared to indium, making it a preferred choice in alloy production where budget constraints are critical. Indium's scarcity and higher extraction costs result in significantly higher market prices, affecting the overall alloy manufacturing expenses. The greater availability of gallium supports its widespread use in electronics and semiconductor applications, while indium remains limited to niche uses due to its cost and supply constraints.
Performance in High-Temperature Environments
Gallium alloys exhibit superior thermal stability and maintain structural integrity at temperatures up to 500degC, making them ideal for high-temperature applications. Indium alloys, while offering excellent corrosion resistance, tend to lose mechanical strength above 300degC, limiting their use in extreme thermal environments. Performance metrics indicate gallium-based alloys outperform indium alternatives in retaining tensile strength and thermal conductivity under prolonged high-temperature exposure.
Alloy Compatibility with Other Metals
Gallium exhibits excellent alloy compatibility with aluminum, copper, and zinc, forming low-melting point alloys ideal for soldering and thermal interface applications. Indium alloys well with metals such as silver, gold, and lead, providing superior ductility and corrosion resistance in electronic and cryogenic environments. Both metals enable customized alloy properties by adjusting composition ratios, but gallium's tendency to embrittle aluminum requires careful handling in structural uses.
Future Trends and Research Directions
Gallium and indium alloys are gaining prominence due to their unique low melting points and high electrical conductivity, crucial for flexible electronics and thermal interface materials. Future research emphasizes improving their mechanical stability, oxidation resistance, and cost-efficiency while exploring nanoscale alloying techniques to enhance performance for wearable devices and soft robotics. Emerging trends highlight gallium-indium combinations in liquid metal applications and energy harvesting technologies, driven by advances in materials science and nanotechnology.
Infographic: Gallium vs Indium for Alloy
 azmater.com
azmater.com