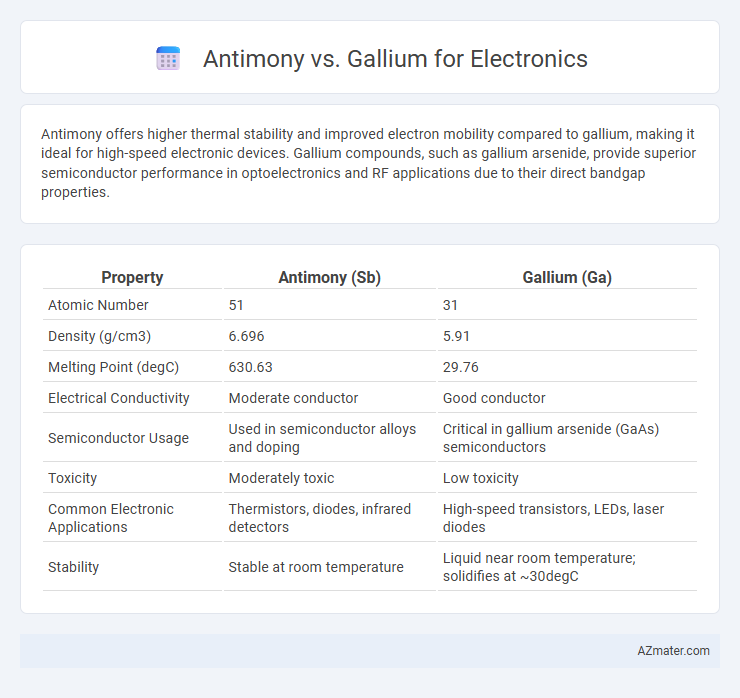
Antimony offers higher thermal stability and improved electron mobility compared to gallium, making it ideal for high-speed electronic devices. Gallium compounds, such as gallium arsenide, provide superior semiconductor performance in optoelectronics and RF applications due to their direct bandgap properties.
Table of Comparison
| Property | Antimony (Sb) | Gallium (Ga) |
|---|---|---|
| Atomic Number | 51 | 31 |
| Density (g/cm3) | 6.696 | 5.91 |
| Melting Point (degC) | 630.63 | 29.76 |
| Electrical Conductivity | Moderate conductor | Good conductor |
| Semiconductor Usage | Used in semiconductor alloys and doping | Critical in gallium arsenide (GaAs) semiconductors |
| Toxicity | Moderately toxic | Low toxicity |
| Common Electronic Applications | Thermistors, diodes, infrared detectors | High-speed transistors, LEDs, laser diodes |
| Stability | Stable at room temperature | Liquid near room temperature; solidifies at ~30degC |
Introduction to Antimony and Gallium in Electronics
Antimony is a metalloid widely used in semiconductor technology to create diodes, infrared detectors, and hall-effect devices due to its ability to form stable compounds with elements like sulfur and tellurium. Gallium plays a critical role in electronics through its compound semiconductor forms, such as gallium arsenide (GaAs), which offers superior electron mobility and high-frequency performance in integrated circuits and optoelectronics. Both elements enhance electronic device efficiency, with antimony improving thermoelectric properties and gallium driving advances in high-speed and optoelectronic applications.
Elemental Properties and Characteristics
Antimony exhibits a higher atomic number (51) and density (6.697 g/cm3) compared to gallium's atomic number (31) and density (5.91 g/cm3), influencing their electrical conductivity and thermal stability in electronic applications. Gallium's low melting point (29.76degC) and excellent semiconductor properties, particularly in gallium arsenide (GaAs), make it ideal for high-speed and optoelectronic devices, while antimony is valued for its role as a dopant and its ability to improve the performance of semiconductors like lead-acid batteries and infrared detectors. The distinct elemental characteristics, including antimony's metalloid nature and gallium's post-transition metal classification, determine their respective functionalities and suitability in diverse electronic components.
Electronic Band Structure Comparison
Antimony exhibits a narrow indirect band gap of approximately 0.14 eV, making it suitable for semimetal applications with moderate electron mobility, while gallium, commonly in compounds like gallium arsenide (GaAs), features a direct band gap around 1.42 eV, facilitating efficient electron-hole recombination for optoelectronics. The electronic band structure of antimony reveals overlapping conduction and valence bands indicative of semimetallic behavior, contrasting with gallium-based semiconductors that have well-defined band edges critical for high-speed electronic and photovoltaic devices. Consequently, gallium compounds dominate in applications requiring high electron mobility and direct transition processes, while antimony's band structure supports niche roles in thermoelectrics and infrared detectors.
Material Availability and Sustainability
Antimony is more abundant in the Earth's crust, with significant deposits primarily in China, making it relatively accessible for electronics manufacturing; however, concerns about its toxic extraction processes impact sustainability. Gallium is rarer, a byproduct of aluminum and zinc mining, with limited sources mostly in China and Germany, challenging its long-term availability for electronic applications. Both materials require careful resource management, but gallium's recycling potential and lower environmental toxicity offer advantages for sustainable electronics development.
Electrical Conductivity and Performance
Antimony exhibits moderate electrical conductivity, making it useful as a dopant in semiconductors to enhance n-type conductivity, while gallium offers superior electrical conductivity, especially in compound semiconductors like gallium arsenide (GaAs), which features high electron mobility and efficient charge carrier transport. Gallium-based semiconductors provide faster electron velocity and improved performance in high-frequency and optoelectronic devices compared to antimony-doped materials. Consequently, gallium is preferred in advanced electronics requiring high speed and efficiency, whereas antimony is primarily utilized to tailor electrical properties in silicon-based technologies.
Compatibility with Semiconductor Technologies
Antimony exhibits strong compatibility with III-V semiconductor technologies, often used as a dopant in indium antimonide for high-speed electronics and infrared detectors, enhancing electron mobility and thermal stability. Gallium, particularly in gallium arsenide and gallium nitride, dominates the semiconductor industry due to superior electron velocity and efficient optoelectronic properties, crucial for high-frequency and power devices. Both elements integrate well within different semiconductor fabrication processes but serve distinctly tailored applications depending on performance requirements and device architecture.
Applications in Modern Electronics
Antimony's unique semiconducting properties make it essential in thermoelectric devices, infrared detectors, and diodes, leveraging its ability to enhance electronic performance through improved charge carrier mobility. Gallium, particularly in the form of gallium arsenide (GaAs), dominates high-frequency and optoelectronic applications due to its superior electron mobility and direct bandgap, enabling faster transistors, efficient LEDs, and solar cells. Both elements are critical in modern electronics, with antimony improving thermal stability and gallium optimizing speed and efficiency in advanced communication systems and photonic devices.
Environmental Impact and Safety Considerations
Antimony and gallium both play critical roles in electronics, with antimony widely used in flame retardants and semiconductors, while gallium is essential for high-speed devices and LEDs. Environmental impact of antimony includes toxicity concerns and persistence in ecosystems, whereas gallium presents lower toxicity but challenges in sustainable extraction and recycling. Safety considerations emphasize strict handling protocols for antimony due to its potential carcinogenicity, while gallium requires caution to prevent environmental contamination during mining and disposal processes.
Cost Analysis and Market Trends
Antimony exhibits a lower cost per kilogram compared to gallium, making it a more economical choice for large-scale electronic applications such as infrared detectors and diodes. Gallium demand is rising sharply due to its crucial role in advanced semiconductors and LED manufacturing, driving higher market prices and supply constraints. Market trends indicate gallium's premium valuation persists due to its integration in cutting-edge electronics, while antimony remains favored for cost-sensitive components and emerging markets.
Future Prospects and Research Directions
Antimony and gallium are essential elements in semiconductor technology, with antimony showing promise for high-speed electronics and infrared detectors due to its unique electron mobility and thermal properties. Research is increasingly focused on integrating antimony-based compounds like indium antimonide (InSb) into next-generation spintronic devices and quantum computing, driven by efforts to exploit its narrow bandgap and strong spin-orbit coupling. Gallium, especially in gallium nitride (GaN) and gallium arsenide (GaAs) compounds, remains pivotal for power electronics and optoelectronics, with ongoing advancements targeting improved efficiency, thermal management, and scalability for 5G networks and electric vehicle applications.
Infographic: Antimony vs Gallium for Electronics
 azmater.com
azmater.com