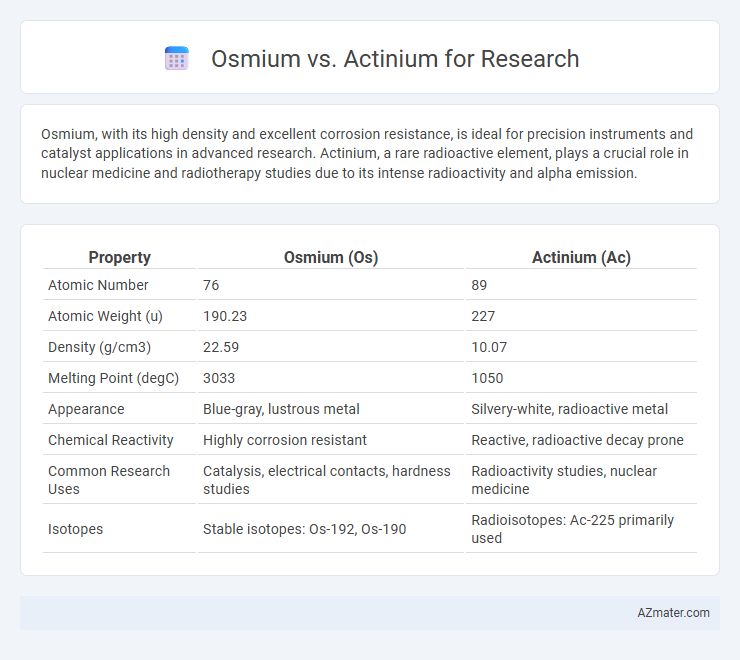
Osmium, with its high density and excellent corrosion resistance, is ideal for precision instruments and catalyst applications in advanced research. Actinium, a rare radioactive element, plays a crucial role in nuclear medicine and radiotherapy studies due to its intense radioactivity and alpha emission.
Table of Comparison
| Property | Osmium (Os) | Actinium (Ac) |
|---|---|---|
| Atomic Number | 76 | 89 |
| Atomic Weight (u) | 190.23 | 227 |
| Density (g/cm3) | 22.59 | 10.07 |
| Melting Point (degC) | 3033 | 1050 |
| Appearance | Blue-gray, lustrous metal | Silvery-white, radioactive metal |
| Chemical Reactivity | Highly corrosion resistant | Reactive, radioactive decay prone |
| Common Research Uses | Catalysis, electrical contacts, hardness studies | Radioactivity studies, nuclear medicine |
| Isotopes | Stable isotopes: Os-192, Os-190 | Radioisotopes: Ac-225 primarily used |
Introduction to Osmium and Actinium in Research
Osmium, a dense transition metal with atomic number 76, is prized in research for its exceptional hardness, high density, and catalytic properties, making it valuable in material science and chemical applications. Actinium, with atomic number 89, is a rare radioactive element extensively studied in nuclear medicine and radiotherapy due to its alpha-emitting isotopes, particularly Actinium-225. Both elements play crucial roles in advancing scientific knowledge, with Osmium driving innovations in durable materials and catalysis, while Actinium enables targeted cancer treatments and nuclear research.
Chemical Properties: Osmium vs Actinium
Osmium exhibits exceptional chemical stability with a high density of 22.59 g/cm3 and primarily forms compounds in the +4 and +8 oxidation states, making it highly resistant to corrosion and ideal for catalytic applications. Actinium, a radioactive actinide element, exists predominantly in the +3 oxidation state and displays significant radioactivity with a half-life of 21.7 years, affecting its chemical behavior and handling in research. The contrasting stability and radioactivity of osmium and actinium define their distinct roles in chemical research, where osmium's inertness supports material science studies and actinium's radioactivity informs nuclear chemistry investigations.
Abundance and Availability for Scientific Studies
Osmium, a dense transition metal with an abundance of approximately 0.001 ppm in the Earth's crust, is relatively more accessible for scientific studies compared to actinium, which is a rare, radioactive actinide with an abundance of about 1x10-10 ppm. The limited natural availability and the high radioactivity of actinium pose significant challenges for researchers in terms of sourcing, handling, and long-term study. Osmium's availability allows for broader experimental applications in catalysis and material science, whereas actinium research is typically restricted to specialized nuclear and radiochemical investigations.
Handling and Safety Concerns
Osmium requires careful handling due to its toxicity, especially osmium tetroxide, which is volatile and highly toxic when inhaled. Actinium poses significant radiological hazards, necessitating strict shielding and contamination control because of its intense radioactivity and alpha particle emission. Laboratories working with either element must implement specialized safety protocols, including proper ventilation, protective equipment, and radiation monitoring to mitigate health risks effectively.
Applications in Modern Research
Osmium exhibits exceptional density and catalytic properties, making it valuable in applications such as high-precision instruments, electron microscopy, and electrocatalysis for fuel cells. Actinium, known for its radioactivity, plays a critical role in targeted alpha-particle cancer therapy and nuclear medicine research, particularly in developing radiopharmaceuticals. Modern research leverages osmium's durability for material science innovations and actinium's radioisotopes for advancing oncological treatments.
Radioactivity: Implications for Laboratory Use
Osmium, a dense transition metal with negligible radioactivity, offers safer handling and minimal radiation shielding requirements in laboratory research compared to Actinium, a highly radioactive actinide emitting alpha particles. The intense radioactivity of Actinium necessitates stringent safety protocols, specialized containment, and continuous monitoring to mitigate radiation exposure risks during experimental procedures. Consequently, Osmium is often preferred for studies where low radioactivity is essential, whereas Actinium's properties are exploited primarily in nuclear medicine and radiopharmaceutical research despite its handling challenges.
Analytical Techniques for Osmium and Actinium
Osmium is often analyzed using techniques such as inductively coupled plasma mass spectrometry (ICP-MS) and X-ray absorption spectroscopy (XAS) to determine its isotopic compositions and oxidation states, crucial for geochemical and medical research. Actinium, due to its radioactivity and rarity, requires specialized methods like alpha spectrometry and liquid scintillation counting for precise activity measurement and radiochemical analysis. Both elements demand rigorous sample preparation and containment protocols to accurately characterize their chemical and nuclear properties in research applications.
Comparative Costs and Economic Considerations
Osmium, a rare and dense transition metal, incurs high costs due to its scarcity and complex extraction processes, often limiting its use to specialized research applications such as catalysis and materials science. Actinium, a radioactive actinide, presents significant economic challenges related to its limited availability, expensive handling requirements, and regulatory compliance for safe use in nuclear medicine and radiopharmaceutical research. The comparative costs between osmium and actinium heavily influence research feasibility, with osmium offering higher material costs but fewer regulatory burdens, while actinium demands substantial investment in safety infrastructure and licensing.
Environmental Impact and Sustainability
Osmium, a dense transition metal, poses significant environmental risks due to its rarity and the toxic osmium tetroxide it can form, complicating safe handling and disposal in research applications. Actinium, an actinide with limited natural occurrence and intense radioactivity, presents sustainability challenges linked to radioactive waste management and potential contamination of ecosystems. Research involving these elements requires stringent protocols to mitigate environmental impact while exploring sustainable extraction and utilization methods.
Future Prospects in Scientific Research
Osmium's exceptional density and catalytic properties position it as a critical element for advancing material science and high-precision instruments in future research. Actinium's radioactivity and potential use in targeted alpha therapy highlight its transformative prospects in nuclear medicine and radiopharmaceutical development. Both elements offer unique pathways for innovation, with osmium influencing technological applications and actinium driving breakthroughs in cancer treatment and nuclear science.
Infographic: Osmium vs Actinium for Research
 azmater.com
azmater.com