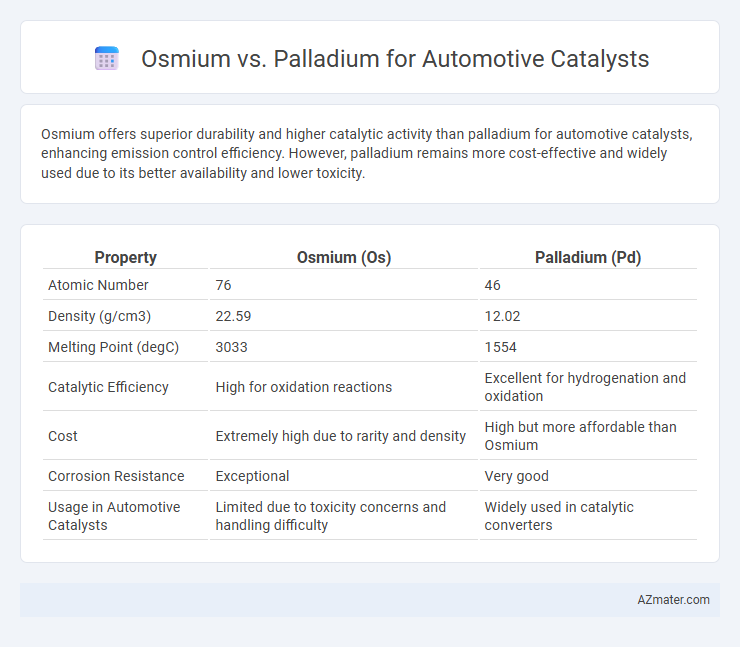
Osmium offers superior durability and higher catalytic activity than palladium for automotive catalysts, enhancing emission control efficiency. However, palladium remains more cost-effective and widely used due to its better availability and lower toxicity.
Table of Comparison
| Property | Osmium (Os) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 76 | 46 |
| Density (g/cm3) | 22.59 | 12.02 |
| Melting Point (degC) | 3033 | 1554 |
| Catalytic Efficiency | High for oxidation reactions | Excellent for hydrogenation and oxidation |
| Cost | Extremely high due to rarity and density | High but more affordable than Osmium |
| Corrosion Resistance | Exceptional | Very good |
| Usage in Automotive Catalysts | Limited due to toxicity concerns and handling difficulty | Widely used in catalytic converters |
Introduction to Osmium and Palladium in Automotive Catalysts
Osmium and palladium serve distinct roles in automotive catalysts due to their unique chemical properties and catalytic efficiencies. Palladium is widely used in catalytic converters for its excellent oxidation and hydrogenation capabilities, contributing to the reduction of harmful emissions such as carbon monoxide and hydrocarbons. Osmium, though less common, is noted for its high durability and resistance to corrosion, making it a potential candidate for specialized catalytic applications in harsh automotive environments.
Chemical Properties: Osmium vs Palladium
Osmium exhibits exceptional chemical stability and high resistance to oxidation, making it effective in harsh catalytic environments, whereas palladium offers superior catalytic activity and selectivity for hydrogenation and oxidation reactions in automotive catalysts. Palladium's chemical properties include excellent adsorption of hydrocarbons and oxygen, facilitating efficient emission control, while osmium's dense atomic structure contributes to its durability under extreme conditions. Both metals serve critical roles, but palladium remains the preferred choice due to its optimal balance of reactivity and stability in catalytic converters.
Catalytic Efficiency in Emission Control
Osmium and palladium differ significantly in catalytic efficiency for automotive emission control, with palladium being preferred due to its superior ability to catalyze oxidation of hydrocarbons and carbon monoxide effectively at lower temperatures. Osmium, although a platinum-group metal, exhibits lower catalytic activity and stability under the harsh conditions within catalytic converters, limiting its practical use. Palladium's higher resistance to poisoning and better performance in three-way catalysts enhances its role in reducing nitrogen oxides, hydrocarbons, and carbon monoxide emissions efficiently.
Durability and Longevity in Automotive Applications
Osmium exhibits exceptional durability and corrosion resistance, enhancing the longevity of automotive catalysts exposed to high-temperature exhaust gases. Palladium is widely used due to its superior catalytic efficiency but may experience deactivation over time under harsh thermal cycling conditions. Osmium's higher melting point and resistance to sintering make it a promising material for improving the lifespan of catalytic converters in aggressive automotive environments.
Cost Comparison and Market Availability
Osmium is significantly rarer and more expensive than palladium, with prices often exceeding palladium by several multiples due to its scarcity and complex extraction processes. Palladium dominates the automotive catalyst market because of its balanced cost, high availability from established mining operations primarily in Russia and South Africa, and proven catalytic efficiency. Market trends indicate that palladium remains the preferred choice for catalytic converters, while osmium's limited availability and steep cost hinder its widespread adoption despite its catalytic potential.
Environmental Impact and Sustainability
Osmium and palladium play distinct roles in automotive catalysts, with palladium widely preferred due to its higher abundance and lower environmental mining impact compared to osmium. Palladium-based catalysts efficiently reduce harmful emissions like nitrogen oxides, carbon monoxide, and hydrocarbons, contributing significantly to air quality improvements and regulatory compliance in automotive sectors. While osmium has potential catalytic properties, its rarity and toxic byproducts pose significant sustainability challenges, making palladium the more environmentally responsible choice for catalytic converters.
Toxicity and Safety Considerations
Osmium and palladium differ significantly in toxicity and safety profiles when used in automotive catalysts. Osmium, particularly in its volatile tetroxide form, is highly toxic and poses severe health risks through inhalation and skin contact. Palladium exhibits lower toxicity, making it safer for catalytic converters with reduced environmental and occupational hazards.
Recent Advancements in Catalyst Technology
Recent advancements in automotive catalyst technology highlight the increased interest in osmium and palladium due to their unique catalytic properties. Osmium offers exceptional durability and resistance to high-temperature sintering, making it ideal for long-lasting catalytic converters, while palladium remains favored for its high efficiency in oxidizing hydrocarbons and nitrogen oxides at lower operating temperatures. Innovations in nanostructured alloys and support materials have enhanced the dispersion and activity of both metals, leading to improved emission control and reduced reliance on more expensive platinum group metals.
Future Trends: Osmium vs Palladium Adoption
Palladium remains the dominant catalyst metal in automotive catalytic converters due to its proven performance and supply stability, but rising prices and supply risks have spurred interest in alternative materials like osmium. Osmium offers exceptional catalytic properties and durability under high-temperature conditions, positioning it as a potential future candidate for automotive catalyst applications. Advances in nanotechnology and alloy development could drive increased osmium adoption, although its scarcity and toxicity challenges must be addressed to compete with palladium's entrenched market presence.
Conclusion: Choosing the Optimal Automotive Catalyst
Osmium offers exceptional catalytic durability and resistance to high temperatures, making it suitable for long-lasting automotive catalysts, while palladium excels in cost-effectiveness and efficient oxidation of harmful emissions. The optimal automotive catalyst choice depends on balancing performance requirements with economic constraints, where palladium remains dominant due to its widespread availability and proven effectiveness. Future advancements in catalyst technology may leverage osmium's superior properties, but currently, palladium provides the best performance-to-cost ratio for mainstream automotive applications.
Infographic: Osmium vs Palladium for Automotive Catalyst
 azmater.com
azmater.com