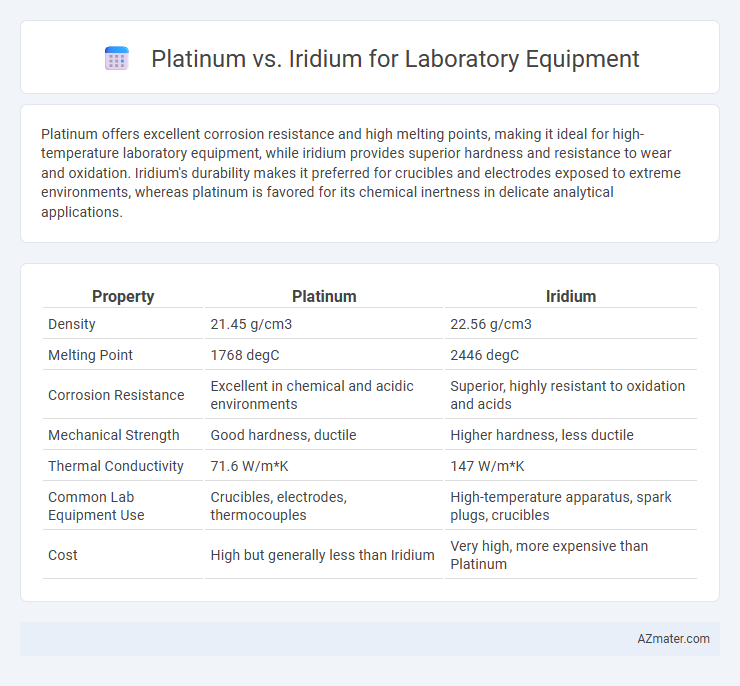
Platinum offers excellent corrosion resistance and high melting points, making it ideal for high-temperature laboratory equipment, while iridium provides superior hardness and resistance to wear and oxidation. Iridium's durability makes it preferred for crucibles and electrodes exposed to extreme environments, whereas platinum is favored for its chemical inertness in delicate analytical applications.
Table of Comparison
| Property | Platinum | Iridium |
|---|---|---|
| Density | 21.45 g/cm3 | 22.56 g/cm3 |
| Melting Point | 1768 degC | 2446 degC |
| Corrosion Resistance | Excellent in chemical and acidic environments | Superior, highly resistant to oxidation and acids |
| Mechanical Strength | Good hardness, ductile | Higher hardness, less ductile |
| Thermal Conductivity | 71.6 W/m*K | 147 W/m*K |
| Common Lab Equipment Use | Crucibles, electrodes, thermocouples | High-temperature apparatus, spark plugs, crucibles |
| Cost | High but generally less than Iridium | Very high, more expensive than Platinum |
Introduction to Platinum and Iridium in Laboratory Equipment
Platinum and iridium are highly valued in laboratory equipment for their exceptional corrosion resistance and high melting points, making them ideal for high-temperature applications. Platinum is widely used in crucibles, electrodes, and catalysts due to its excellent chemical stability and electrical conductivity. Iridium offers superior hardness and wear resistance, often alloyed with platinum to enhance durability in demanding laboratory environments.
Key Properties of Platinum
Platinum is highly valued in laboratory equipment for its exceptional corrosion resistance and ability to withstand high temperatures up to 1,768degC, making it ideal for crucibles and electrodes. Its excellent chemical inertness ensures minimal contamination in sensitive experiments, distinguishing it from iridium's higher hardness but lower ductility. The metal's superior malleability and thermal stability provide reliability and durability in scientific applications requiring precise and consistent performance.
Essential Characteristics of Iridium
Iridium exhibits exceptional corrosion resistance and maintains structural integrity at extremely high temperatures, making it ideal for laboratory equipment exposed to harsh chemical environments. Its high density and hardness contribute to durability and resistance to wear, crucial for precise instruments and components. Iridium's superior chemical inertness surpasses platinum, ensuring longevity and reliability in critical laboratory applications.
Chemical Resistance: Platinum vs Iridium
Platinum exhibits exceptional chemical resistance, particularly against acids and oxidizing agents, making it highly suitable for laboratory equipment exposed to harsh chemical environments. Iridium, while also chemically stable, offers superior resistance to corrosion and high-temperature oxidation compared to platinum, especially in aggressive chemical conditions. The choice between platinum and iridium for laboratory applications depends on the specific chemical milieu and temperature, with iridium favored for extreme corrosion resistance and platinum preferred for general chemical durability.
Thermal Stability and Melting Points
Platinum and iridium both exhibit exceptional thermal stability, with platinum melting at approximately 1,768degC and iridium surpassing this at around 2,446degC, making iridium more suitable for high-temperature laboratory applications. Iridium's higher melting point and resistance to oxidation enable it to maintain structural integrity under extreme thermal stress, while platinum offers excellent corrosion resistance and durability in slightly lower temperature ranges. Choosing between these metals depends on the specific thermal demands of laboratory equipment, with iridium preferred for ultra-high temperature processes and platinum favored for applications requiring chemical inertness combined with high thermal endurance.
Durability and Longevity in Lab Applications
Platinum exhibits exceptional corrosion resistance and high melting point, making it ideal for laboratory equipment subjected to intense chemical reactions and elevated temperatures. Iridium, often alloyed with platinum, significantly enhances hardness and wear resistance, extending the lifespan of lab tools under abrasive conditions. The superior durability of platinum-iridium alloys ensures consistent performance and longevity in precision instruments, crucibles, and electrodes used in rigorous scientific environments.
Cost Comparison: Platinum vs Iridium
Platinum typically costs less than iridium, making it a more budget-friendly choice for laboratory equipment requiring high corrosion resistance and durability. Iridium's higher price stems from its greater rarity and exceptional hardness, which enhance its longevity in extreme conditions but increase initial investment costs. Laboratories must balance the premium price of iridium against its extended lifespan and specialized applications compared to the more economical, yet highly effective, platinum.
Common Laboratory Uses of Platinum
Platinum is widely used in laboratory equipment due to its excellent corrosion resistance and high melting point, making it ideal for crucibles, electrodes, and evaporation dishes in chemical experiments. Iridium, although similarly resistant to corrosion and heat, is primarily favored for its hardness in specialized applications like spark plugs and high-precision instruments. Platinum's chemical inertness and durability make it the preferred choice for common laboratory uses, especially in procedures involving strong acids and high-temperature reactions.
Typical Laboratory Applications of Iridium
Iridium's exceptional resistance to corrosion and high melting point make it ideal for crucibles and electrodes in high-temperature laboratory applications, such as furnace operations and electrochemical cells. Typically used in environments requiring durability against aggressive chemicals and extreme heat, iridium is favored for devices like high-precision thermocouples and spark plug electrodes. Its stability under harsh conditions surpasses that of platinum, ensuring reliable performance in specialized analytical and material science equipment.
Choosing the Right Material for Your Laboratory Needs
Platinum and iridium offer distinct advantages for laboratory equipment, with platinum providing excellent corrosion resistance and high thermal conductivity ideal for chemical reactions and high-temperature applications. Iridium excels in extreme durability and resistance to oxidation, making it suitable for crucibles, electrodes, and devices exposed to intense heat or corrosive environments. Selecting the right material depends on your specific laboratory needs, balancing factors such as temperature tolerance, chemical reactivity, and mechanical strength.
Infographic: Platinum vs Iridium for Laboratory Equipment
 azmater.com
azmater.com