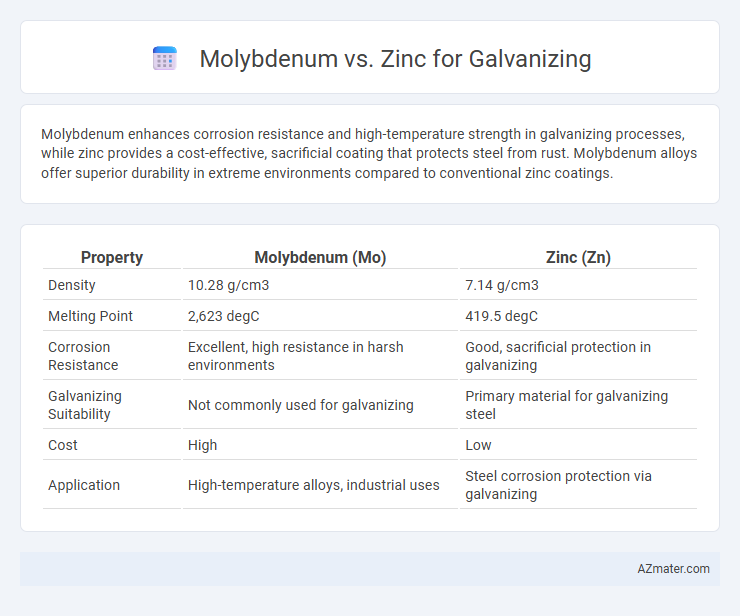
Molybdenum enhances corrosion resistance and high-temperature strength in galvanizing processes, while zinc provides a cost-effective, sacrificial coating that protects steel from rust. Molybdenum alloys offer superior durability in extreme environments compared to conventional zinc coatings.
Table of Comparison
| Property | Molybdenum (Mo) | Zinc (Zn) |
|---|---|---|
| Density | 10.28 g/cm3 | 7.14 g/cm3 |
| Melting Point | 2,623 degC | 419.5 degC |
| Corrosion Resistance | Excellent, high resistance in harsh environments | Good, sacrificial protection in galvanizing |
| Galvanizing Suitability | Not commonly used for galvanizing | Primary material for galvanizing steel |
| Cost | High | Low |
| Application | High-temperature alloys, industrial uses | Steel corrosion protection via galvanizing |
Introduction to Galvanizing: The Role of Alloying Elements
Galvanizing involves coating steel with zinc to prevent rust and corrosion, where alloying elements significantly impact the coating's durability and performance. Molybdenum enhances corrosion resistance and improves the adherence of the zinc layer, providing longer-lasting protection. Zinc, as the primary metal in galvanizing, offers sacrificial protection by corroding preferentially, but alloying with elements like molybdenum optimizes the coating's mechanical properties and resistance to harsh environments.
Overview of Molybdenum and Zinc Properties
Molybdenum exhibits a high melting point of 2,623degC and exceptional corrosion resistance, making it ideal for enhancing steel alloys in galvanizing processes. Zinc, with a melting point of 419.5degC, provides excellent sacrificial corrosion protection by forming a stable oxide layer, preventing rust on steel surfaces. The distinct physical and chemical properties of molybdenum and zinc dictate their specific roles in galvanizing, optimizing durability and resistance in coated metals.
Galvanizing Processes: Where Molybdenum and Zinc Fit
Molybdenum serves as an alloying element in galvanizing steel to enhance corrosion resistance and improve coating adhesion, particularly under high-temperature conditions. Zinc remains the primary metal in hot-dip galvanizing, providing a sacrificial layer that protects steel from rust by oxidizing first. Understanding the specific roles of molybdenum and zinc facilitates optimized galvanizing processes aimed at prolonging the lifespan and durability of metal components.
Corrosion Resistance: Molybdenum vs Zinc
Molybdenum exhibits superior corrosion resistance compared to zinc due to its ability to form stable, protective oxide layers that withstand harsh environmental conditions and high temperatures. Zinc primarily offers sacrificial corrosion protection by corroding itself to protect underlying metals, but it is less effective in highly acidic or alkaline environments. The enhanced durability of molybdenum makes it ideal for applications where long-term resistance to aggressive corrosion is critical.
Mechanical Strength and Durability Comparison
Molybdenum-enhanced galvanizing coatings exhibit superior mechanical strength due to their increased hardness and resistance to deformation under stress compared to zinc-only coatings. The addition of molybdenum significantly improves the durability of galvanized steel by enhancing corrosion resistance and reducing the rate of wear in harsh environments. Zinc provides excellent baseline protection against rust, but molybdenum alloys extend service life by maintaining structural integrity in demanding mechanical applications.
Cost Analysis: Molybdenum vs Zinc in Galvanizing
Molybdenum is significantly more expensive than zinc, making zinc the preferred choice for galvanizing from a cost-efficiency perspective. Zinc offers excellent corrosion resistance at a lower price point, reducing overall production costs in large-scale galvanizing applications. The higher material and processing costs of molybdenum limit its use to specialized applications where enhanced performance justifies the investment.
Environmental Impact and Sustainability Considerations
Molybdenum and zinc both play critical roles in galvanizing processes, with zinc being the primary metal used due to its excellent corrosion resistance and relatively low environmental footprint. Zinc galvanizing offers high sustainability benefits, including full recyclability and lower energy consumption compared to alternative coatings, while molybdenum often serves as an alloying element to enhance steel strength but is less common for direct galvanizing applications. Environmental impact assessments highlight zinc's advantage in reducing steel corrosion without toxic byproducts, whereas molybdenum mining and processing can have higher ecological and resource depletion concerns.
Industrial Applications: Choosing Between Molybdenum and Zinc
Molybdenum offers superior corrosion resistance and high-temperature strength compared to zinc, making it ideal for industrial applications requiring enhanced durability and longevity, such as chemical processing and aerospace components. Zinc remains the preferred choice for galvanizing steel due to its excellent sacrificial protection against rust and cost-effectiveness in construction, automotive, and infrastructure sectors. The selection between molybdenum and zinc depends on balancing environmental exposure, mechanical stress, and budget constraints for optimal industrial performance.
Recent Innovations in Galvanizing Alloys
Recent innovations in galvanizing alloys have explored the addition of molybdenum as a high-performance alternative to zinc, enhancing corrosion resistance and thermal stability in harsh environments. Molybdenum's superior hardness and oxidation resistance improve the lifespan of galvanized steel, especially in industrial and marine applications where zinc alone may degrade more rapidly. Advances in alloying techniques now enable precise control over molybdenum-zinc blends, optimizing coating thickness and adhesion for improved protective performance.
Conclusion: Which is Better for Galvanizing—Molybdenum or Zinc?
Zinc remains the superior choice for galvanizing due to its proven corrosion resistance, cost-effectiveness, and extensive industry adoption. Molybdenum, while offering high corrosion resistance in extreme environments, is significantly more expensive and less practical for wide-scale galvanizing applications. The balance of performance, availability, and economic factors firmly positions zinc as the preferred metal for galvanizing steel.
Infographic: Molybdenum vs Zinc for Galvanizing
 azmater.com
azmater.com