Palladium offers superior corrosion resistance and excellent electrical conductivity for bonding applications, outperforming aluminum's lightweight and cost-effective properties. Aluminum is favored in industries prioritizing weight reduction and cost efficiency but may require surface treatment to enhance bond strength compared to palladium.
Table of Comparison
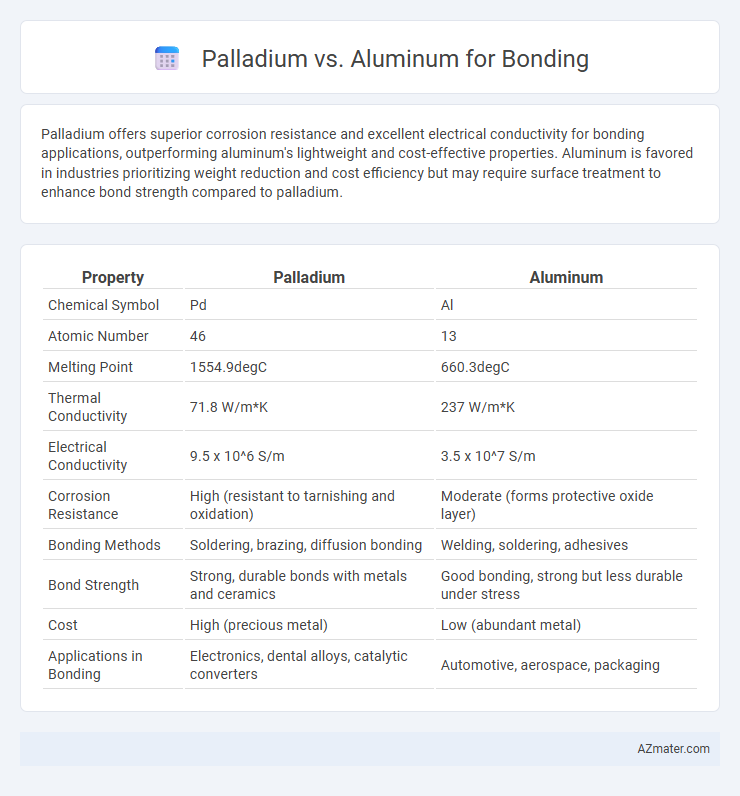
| Property | Palladium | Aluminum |
|---|---|---|
| Chemical Symbol | Pd | Al |
| Atomic Number | 46 | 13 |
| Melting Point | 1554.9degC | 660.3degC |
| Thermal Conductivity | 71.8 W/m*K | 237 W/m*K |
| Electrical Conductivity | 9.5 x 10^6 S/m | 3.5 x 10^7 S/m |
| Corrosion Resistance | High (resistant to tarnishing and oxidation) | Moderate (forms protective oxide layer) |
| Bonding Methods | Soldering, brazing, diffusion bonding | Welding, soldering, adhesives |
| Bond Strength | Strong, durable bonds with metals and ceramics | Good bonding, strong but less durable under stress |
| Cost | High (precious metal) | Low (abundant metal) |
| Applications in Bonding | Electronics, dental alloys, catalytic converters | Automotive, aerospace, packaging |
Introduction to Palladium and Aluminum Bonding
Palladium and aluminum bonding are critical processes in electronics and material science, each offering unique properties for joining metals. Palladium provides excellent corrosion resistance and high electrical conductivity, making it ideal for microelectronic connections. Aluminum bonding is favored for its lightweight, cost-effectiveness, and strong thermal conductivity in automotive and aerospace industries.
Chemical and Physical Properties: Palladium vs Aluminum
Palladium exhibits superior corrosion resistance and higher melting point (1555degC) compared to aluminum's melting point of 660degC, making it ideal for bonding applications requiring durability and thermal stability. Chemically, palladium is a noble metal with minimal reactivity, ensuring long-term bond integrity, while aluminum forms a passive oxide layer that can impact bond strength without proper surface treatment. Physically, palladium has higher density (12.0 g/cm3) and excellent ductility, facilitating strong, reliable metallurgical bonds in electronic and catalytic applications, contrasting with aluminum's lighter weight (2.7 g/cm3) and greater tendency for oxidation.
Bonding Techniques and Processes
Palladium offers superior bonding reliability in electronic applications due to its excellent oxidation resistance and strong metallurgical affinity with various solders, enabling techniques like wire bonding and flip-chip processes with enhanced durability. Aluminum bonding typically relies on ultrasonic wedge bonding or thermosonic bonding, favored for cost efficiency but prone to oxide layer formation that can weaken bond strength without proper surface treatments. Advanced methods such as palladium plating or alloying improve bond interface quality by facilitating consistent metallurgical bonds, reducing resistance and enhancing long-term stability compared to pure aluminum bonds.
Strength and Reliability Comparison
Palladium offers superior bond strength due to its excellent adhesion properties and resistance to oxidation, making it highly reliable in harsh environments. Aluminum, while cost-effective and lightweight, typically forms weaker bonds because of its oxide layer, which can compromise long-term bonding reliability. Applications requiring durable, high-strength joints often favor palladium despite higher costs, whereas aluminum suits less demanding uses with moderate bonding strength.
Corrosion Resistance in Bonding Applications
Palladium exhibits superior corrosion resistance compared to aluminum in bonding applications, making it ideal for environments exposed to humidity, salts, and acidic conditions. Its noble metal properties prevent oxidation and degradation over time, ensuring durable and reliable bonds in electronics and automotive industries. Aluminum, while lightweight and cost-effective, forms an oxide layer that can compromise bond integrity under corrosive stress, requiring additional protective measures.
Electrical and Thermal Conductivity Differences
Palladium exhibits superior electrical conductivity compared to aluminum, making it ideal for high-performance electronic bonding applications where signal integrity is critical. Aluminum, while having lower electrical conductivity, offers better thermal conductivity, which enhances heat dissipation in thermal management applications. The choice between palladium and aluminum for bonding depends on the specific balance required between electrical performance and thermal efficiency.
Cost and Availability: Palladium vs Aluminum
Palladium is significantly more expensive than aluminum, with prices often exceeding thousands of dollars per ounce compared to aluminum's few dollars per pound, impacting overall bonding project costs. Aluminum's wide availability and abundance in the Earth's crust ensure a steady supply and lower material expenses, while palladium, being a rare precious metal, faces supply constraints and price volatility. Cost-effectiveness and material accessibility make aluminum a more practical choice for bonding applications requiring large-scale production or budget-conscious decisions.
Applications in Electronics and Industrial Sectors
Palladium offers superior corrosion resistance and excellent electrical conductivity, making it ideal for high-reliability electronic components such as connectors, capacitors, and semiconductor devices. Aluminum is favored in industrial applications for its lightweight properties and cost-effectiveness in bonding processes like brazing and welding, particularly in automotive and aerospace sectors. The choice between palladium and aluminum depends on performance requirements; palladium is preferred for precision electronic bonding, while aluminum suits large-scale industrial manufacturing.
Environmental Impact and Sustainability
Palladium offers excellent corrosion resistance and durability in bonding applications, but its extraction process involves significant environmental challenges, including high energy consumption and habitat disruption. Aluminum, abundant and lightweight, presents a lower environmental footprint due to easier recycling and less intensive mining practices, enhancing sustainability in manufacturing. Choosing aluminum over palladium can reduce ecological impact while maintaining reliable bonding performance in many industrial uses.
Choosing the Right Material for Bonding Tasks
Palladium offers superior corrosion resistance and excellent electrical conductivity, making it ideal for high-performance bonding tasks in electronics and jewelry. Aluminum provides lightweight strength and cost-effectiveness but requires careful surface preparation to ensure strong adhesion and prevent oxidation issues. Selecting between palladium and aluminum depends on the specific bonding requirements, such as durability, conductivity, and budget constraints.
Infographic: Palladium vs Aluminum for Bonding
 azmater.com
azmater.com