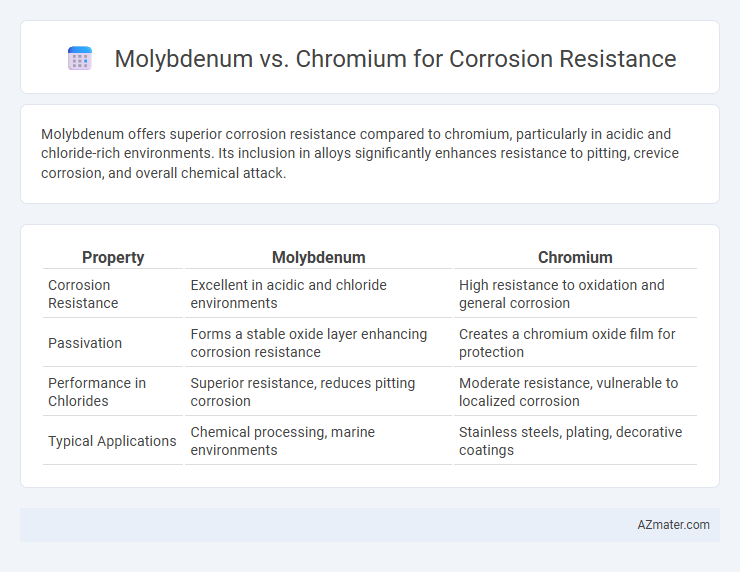
Molybdenum offers superior corrosion resistance compared to chromium, particularly in acidic and chloride-rich environments. Its inclusion in alloys significantly enhances resistance to pitting, crevice corrosion, and overall chemical attack.
Table of Comparison
| Property | Molybdenum | Chromium |
|---|---|---|
| Corrosion Resistance | Excellent in acidic and chloride environments | High resistance to oxidation and general corrosion |
| Passivation | Forms a stable oxide layer enhancing corrosion resistance | Creates a chromium oxide film for protection |
| Performance in Chlorides | Superior resistance, reduces pitting corrosion | Moderate resistance, vulnerable to localized corrosion |
| Typical Applications | Chemical processing, marine environments | Stainless steels, plating, decorative coatings |
Introduction to Corrosion Resistance
Corrosion resistance in metals is crucial for maintaining structural integrity and longevity in harsh environments. Molybdenum enhances corrosion resistance by improving the pitting and crevice corrosion performance, especially in chloride-rich conditions, making it ideal for stainless steels used in marine and chemical applications. Chromium forms a passive oxide layer that protects against oxidation and general corrosion, providing a durable barrier in atmospheric and industrial environments.
Chemical Properties of Molybdenum and Chromium
Molybdenum and chromium both enhance corrosion resistance through distinct chemical properties; molybdenum forms stable molybdate ions (MoO4^2-) that improve resistance in chloride-rich environments, while chromium creates a passive oxide layer (Cr2O3) that protects against oxidation and surface degradation. The high electronegativity and oxidation state variability of molybdenum contribute to its effectiveness in harsh, acidic conditions, whereas chromium's ability to form a dense, adherent oxide film offers superior protection against general corrosion and rust. Their complementary chemical behaviors are leveraged in alloys to optimize corrosion resistance across diverse industrial applications.
Role of Molybdenum in Corrosion Prevention
Molybdenum significantly enhances corrosion resistance by improving the passive film stability on stainless steel, particularly in chloride-rich environments. Its presence reduces pitting and crevice corrosion, making alloys containing molybdenum ideal for harsh chemical and marine conditions. Compared to chromium alone, molybdenum's role is critical for preventing localized corrosion and extending alloy lifespan in aggressive corrosive settings.
Chromium’s Mechanism in Corrosion Protection
Chromium enhances corrosion resistance primarily by forming a stable, adherent oxide layer (Cr2O3) on the metal surface that acts as a passive barrier against environmental oxidants. This passive film self-heals upon damage, preventing further oxidation and maintaining the metal's integrity. In comparison, molybdenum improves pitting resistance by promoting localized stability but lacks the broad passive film formation inherent to chromium's corrosion protection mechanism.
Comparing Molybdenum and Chromium in Stainless Steels
Molybdenum significantly enhances the corrosion resistance of stainless steels, particularly in chloride-rich environments, by improving pitting and crevice corrosion resistance. Chromium forms the essential passive oxide layer that protects stainless steel from general corrosion, but its effectiveness can be limited against localized attack without molybdenum. Stainless steels alloyed with both chromium (typically above 10.5%) and molybdenum (usually 2-3%) exhibit superior durability in aggressive conditions, combining chromium's oxide film stability with molybdenum's resistance to chloride-induced corrosion.
Performance in Various Corrosive Environments
Molybdenum exhibits superior corrosion resistance in chloride-rich environments, effectively preventing pitting and crevice corrosion, making it ideal for seawater and chemical processing applications. Chromium enhances passivation by forming a stable oxide layer, offering excellent protection in oxidizing and mildly acidic conditions. When combined, molybdenum and chromium significantly improve alloy durability across diverse corrosive settings, balancing resistance to both localized and general corrosion mechanisms.
Cost and Availability Considerations
Molybdenum offers superior corrosion resistance compared to chromium, especially in chloride-rich environments, but it is generally more expensive due to its limited availability and higher extraction costs. Chromium is more abundant and cost-effective, making it a preferred choice for general corrosion resistance applications where budget constraints are critical. Balancing cost efficiency and performance often leads to the use of chromium in large-scale projects, while molybdenum is selected for specialized, high-corrosion environments.
Industrial Applications and Case Studies
Molybdenum exhibits superior corrosion resistance compared to chromium in aggressive industrial environments such as chemical processing and marine applications, due to its ability to enhance pitting and crevice corrosion resistance in stainless steels and alloys. Industrial case studies demonstrate that alloys containing 2-6% molybdenum significantly outperform chromium-rich counterparts in resisting chloride-induced corrosion and high-temperature oxidation. For example, in petrochemical plants and desalination systems, molybdenum-enhanced materials extend equipment lifespan and reduce maintenance costs by minimizing localized corrosion damage.
Environmental Impact and Sustainability
Molybdenum enhances corrosion resistance by forming stable passive layers, reducing the need for frequent replacements and lowering environmental waste. Chromium, while effective in corrosion protection, poses concerns due to its toxic hexavalent forms that can contaminate soil and water during manufacturing and disposal processes. Sustainable choices favor molybdenum alloys for their longer lifecycle and lower ecological footprint, contributing to reduced resource consumption and pollution.
Choosing the Right Element for Optimal Corrosion Resistance
Molybdenum offers superior corrosion resistance in acidic and chloride-rich environments due to its ability to enhance passive film stability and prevent pitting, making it ideal for chemical processing and marine applications. Chromium provides excellent general corrosion resistance by forming a stable, protective chromium oxide layer, which is essential for stainless steel's durability in oxidizing environments. Selecting the right element depends on the specific environmental conditions, with molybdenum favored for aggressive, localized corrosion scenarios and chromium preferred for broad-spectrum corrosion protection.
Infographic: Molybdenum vs Chromium for Corrosion Resistance
 azmater.com
azmater.com