High-entropy alloys exhibit superior hydrogen storage capacity and structural stability compared to palladium, which faces challenges like high cost and limited absorption kinetics. Advanced high-entropy alloys offer enhanced durability and faster hydrogen diffusion rates, making them more efficient for long-term hydrogen storage applications.
Table of Comparison
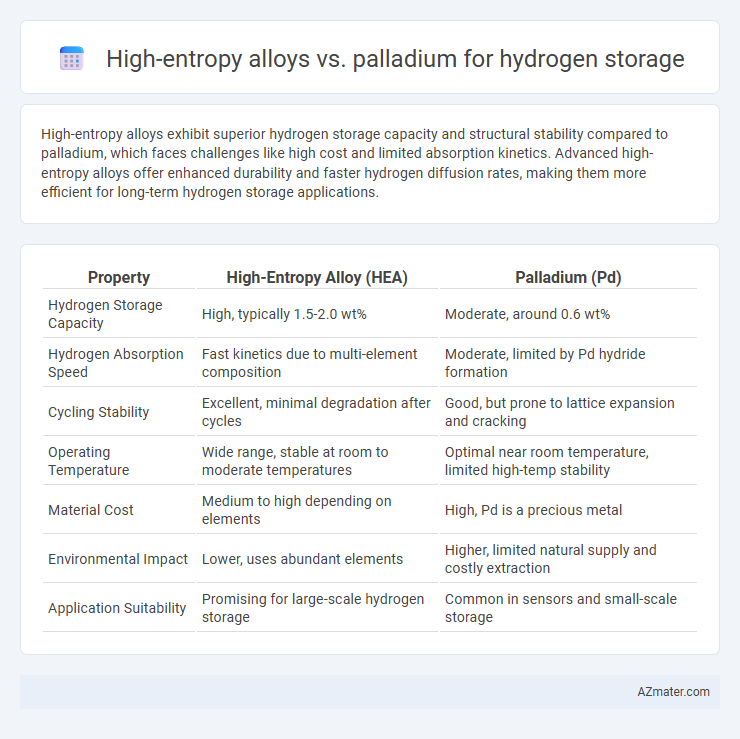
| Property | High-Entropy Alloy (HEA) | Palladium (Pd) |
|---|---|---|
| Hydrogen Storage Capacity | High, typically 1.5-2.0 wt% | Moderate, around 0.6 wt% |
| Hydrogen Absorption Speed | Fast kinetics due to multi-element composition | Moderate, limited by Pd hydride formation |
| Cycling Stability | Excellent, minimal degradation after cycles | Good, but prone to lattice expansion and cracking |
| Operating Temperature | Wide range, stable at room to moderate temperatures | Optimal near room temperature, limited high-temp stability |
| Material Cost | Medium to high depending on elements | High, Pd is a precious metal |
| Environmental Impact | Lower, uses abundant elements | Higher, limited natural supply and costly extraction |
| Application Suitability | Promising for large-scale hydrogen storage | Common in sensors and small-scale storage |
Introduction to Hydrogen Storage Materials
High-entropy alloys (HEAs) and palladium represent advanced materials for hydrogen storage due to their exceptional absorption and diffusion properties. Palladium offers high hydrogen solubility and rapid absorption kinetics but suffers from high cost and limited structural diversity. High-entropy alloys combine multiple principal elements, providing tunable microstructures and enhanced hydrogen storage capacity with improved mechanical stability and resistance to embrittlement.
Overview of High-Entropy Alloys (HEAs)
High-entropy alloys (HEAs) are advanced metallic materials composed of five or more principal elements in near-equiatomic ratios, offering exceptional structural stability and tunable properties. Their unique multi-element composition results in a high configurational entropy, enhancing hydrogen diffusion and absorption capacities compared to conventional palladium-based storage systems. HEAs exhibit promising hydrogen storage performance through improved resistance to hydrogen embrittlement and superior kinetics, making them a competitive alternative to palladium for energy applications.
Palladium: The Classic Hydrogen Storage Material
Palladium remains the classic hydrogen storage material due to its exceptional ability to absorb and release hydrogen at ambient temperatures, with a hydrogen-to-metal atomic ratio reaching up to 0.7. Its face-centered cubic (FCC) crystal structure facilitates rapid hydrogen diffusion and reversible hydride formation, making it a benchmark for hydrogen storage performance. However, challenges such as high cost and susceptibility to mechanical degradation under cycling highlight the ongoing interest in high-entropy alloys (HEAs) as alternative hydrogen storage materials with tunable properties and enhanced durability.
Hydrogen Absorption Mechanisms in HEAs vs Palladium
High-entropy alloys (HEAs) exhibit hydrogen absorption through multi-element lattice distortion that creates diverse interstitial sites, enhancing hydrogen solubility and diffusion compared to palladium's predominantly face-centered cubic (FCC) lattice which absorbs hydrogen via well-defined octahedral and tetrahedral sites. HEAs benefit from synergistic effects among constituent elements, leading to tailored electronic structures that improve hydrogen binding energy and storage capacity, whereas palladium's absorption is limited by phase transformations such as alpha to beta hydride phases. This contrast in mechanisms makes HEAs promising for high-capacity, stable hydrogen storage, while palladium remains a benchmark due to its fast absorption kinetics and reversible hydrogen uptake under moderate conditions.
Storage Capacity Comparison: HEAs vs Palladium
High-entropy alloys (HEAs) demonstrate superior hydrogen storage capacity compared to palladium due to their multi-element compositions creating abundant interstitial sites for hydrogen absorption. Palladium, while highly efficient in selectively absorbing hydrogen, is limited by its lower storage capacity and higher cost. Research indicates that tailored HEAs can achieve storage capacities exceeding palladium's typical 0.6 wt%, making them promising candidates for scalable hydrogen storage applications.
Thermodynamic Performance and Kinetics
High-entropy alloys (HEAs) exhibit superior thermodynamic stability and tunable hydrogen absorption enthalpy compared to palladium, enabling enhanced hydrogen storage capacity at moderate temperatures and pressures. Palladium offers rapid hydrogen absorption and desorption kinetics due to its excellent catalytic properties and favorable lattice structure but suffers from phase transitions that reduce long-term stability. The balance between thermodynamic performance and kinetics makes HEAs promising candidates for efficient hydrogen storage systems, surpassing palladium's limitations in cyclic durability and capacity retention.
Material Stability and Cycling Durability
High-entropy alloys (HEAs) demonstrate superior material stability under hydrogen absorption and desorption cycles compared to palladium, due to their multi-element compositional complexity that reduces phase transformation and embrittlement. Palladium, while exhibiting high hydrogen storage capacity, suffers from lattice expansion and pulverization over repeated cycling, leading to decreased durability. Advanced HEAs maintain structural integrity and reversible hydrogen storage performance over extensive cycles, making them promising candidates for long-term hydrogen storage applications.
Cost and Availability Considerations
High-entropy alloys offer a cost-effective alternative to palladium for hydrogen storage due to their lower raw material expenses and abundant elemental composition, unlike palladium, which is scarce and highly priced on the global market. The widespread availability of constituent elements in high-entropy alloys supports scalable production, whereas palladium's limited supply restricts large-scale applications. These economic and resource factors make high-entropy alloys more viable for sustainable hydrogen storage solutions.
Real-World Applications and Scalability
High-entropy alloys (HEAs) exhibit superior hydrogen storage capacity and enhanced resistance to embrittlement compared to palladium, making them promising candidates for scalable hydrogen storage in fuel cell vehicles and industrial applications. Palladium's high cost and limited availability restrict its use primarily to small-scale or niche hydrogen purification and sensing roles. The tunable composition and abundant element selection in HEAs enable cost-effective mass production and customization for diverse real-world hydrogen storage systems.
Future Prospects in Hydrogen Storage Technologies
High-entropy alloys (HEAs) offer promising future prospects for hydrogen storage technologies due to their exceptional structural stability and tunable hydrogen absorption properties, surpassing traditional palladium-based materials in capacity and durability. The multi-element composition of HEAs enables enhanced resistance to hydrogen embrittlement and improved kinetics, addressing key limitations of palladium such as high cost and limited absorption rates. Ongoing research into optimizing HEA compositions aims to develop scalable, cost-effective hydrogen storage systems critical for advancing clean energy applications.
Infographic: High-entropy alloy vs Palladium for Hydrogen Storage
 azmater.com
azmater.com