Palladium and Rhodium are critical catalysts in automotive catalytic converters, with Palladium primarily used for gasoline engines and Rhodium essential for reducing nitrogen oxides in diesel emissions. Refining Rhodium is more complex and costly due to its rarity and higher melting point compared to Palladium, impacting overall market prices and industrial demand.
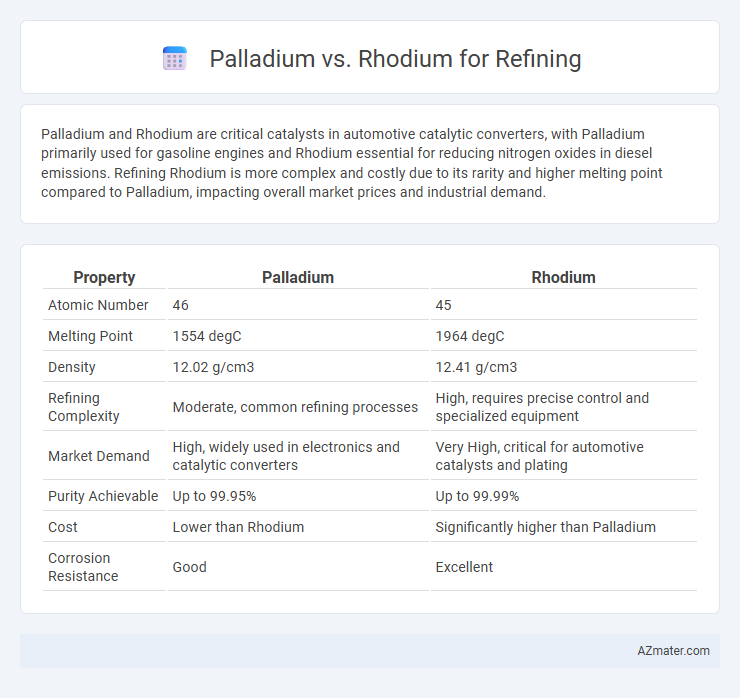
Table of Comparison
| Property | Palladium | Rhodium |
|---|---|---|
| Atomic Number | 46 | 45 |
| Melting Point | 1554 degC | 1964 degC |
| Density | 12.02 g/cm3 | 12.41 g/cm3 |
| Refining Complexity | Moderate, common refining processes | High, requires precise control and specialized equipment |
| Market Demand | High, widely used in electronics and catalytic converters | Very High, critical for automotive catalysts and plating |
| Purity Achievable | Up to 99.95% | Up to 99.99% |
| Cost | Lower than Rhodium | Significantly higher than Palladium |
| Corrosion Resistance | Good | Excellent |
Introduction to Palladium and Rhodium
Palladium and rhodium are both precious metals in the platinum group, extensively used in catalytic converters, electronics, and jewelry due to their outstanding corrosion resistance and catalytic properties. Palladium is valued for its excellent hydrogen absorption and versatility in applications, whereas rhodium is prized for its exceptional reflectivity and ability to withstand extreme temperatures. Refining these metals requires specialized techniques to recover their high purity, with palladium focusing on electrochemical refining and rhodium involving complex chemical separation processes.
Chemical Properties Comparison
Palladium and rhodium both belong to the platinum group metals and exhibit excellent resistance to oxidation and corrosion, but palladium has a lower melting point (1555degC) compared to rhodium's melting point of 1964degC. Chemically, palladium is more versatile in catalytic applications due to its ability to absorb hydrogen up to 900 times its volume, whereas rhodium is valued for its superior catalytic activity in high-temperature environments and exceptional hardness. In refining processes, palladium's chemical stability allows it to effectively catalyze hydrogenation reactions, while rhodium's inertness and resistance to acids make it ideal for use in processes demanding extreme durability.
Industrial Applications in Refining
Palladium and rhodium play crucial roles in industrial refining, particularly in catalytic converters and emission control systems. Palladium excels in hydrogenation and dehydrogenation processes due to its high catalytic activity and resistance to sulfur poisoning. Rhodium is preferred for its superior performance in nitric acid production and three-way catalytic converters, offering enhanced nitrogen oxide reduction and higher thermal stability.
Cost and Market Value Analysis
Palladium typically commands a higher market value due to its widespread demand in automotive catalytic converters, while rhodium's price exhibits greater volatility influenced by its rarity and limited production. Refining costs for rhodium are generally higher because of its complex extraction process and lower occurrence in ores compared to palladium. Market analysis indicates palladium's steady demand supports more predictable refining margins, whereas rhodium's price spikes create opportunities for higher short-term profitability despite increased processing challenges.
Efficiency in Precious Metals Recovery
Palladium excels in refining due to its high catalytic activity and selective absorption properties, enabling efficient recovery of precious metals such as gold and platinum. Rhodium offers superior resistance to corrosion and oxidation, which enhances the longevity of refining catalysts and maintains process stability during high-temperature operations. While palladium delivers faster reaction rates, rhodium's durability can lead to a more sustainable recovery process in complex refining environments.
Environmental Impact of Refining Each Metal
Refining palladium generates fewer toxic emissions compared to rhodium, as palladium processing typically involves less use of hazardous chemicals and lower energy consumption. Rhodium refining releases more harmful pollutants, including nitrogen oxides and sulfur compounds, due to its complex and energy-intensive extraction methods. These environmental concerns make palladium a relatively more sustainable option for jewelry and industrial applications when considering the impact of refining processes.
Advantages of Palladium in Refining
Palladium offers several advantages in refining due to its exceptional catalytic properties and resistance to corrosion, making it highly efficient for hydrogenation processes. Its lower cost compared to rhodium allows for more economical scaling in industrial applications without compromising performance. Palladium's ability to operate effectively under milder conditions reduces energy consumption and enhances overall process sustainability.
Advantages of Rhodium in Refining
Rhodium offers superior corrosion resistance and higher melting point compared to palladium, making it highly advantageous in refining processes involving extreme conditions. Its exceptional catalytic properties enhance the efficiency of refining operations, particularly in the automotive and chemical industries. Rhodium's rarity and durability contribute to improved longevity and value retention in refining equipment and catalysts.
Challenges and Limitations of Both Metals
Palladium refining faces challenges due to its sensitivity to alloy composition variations and contamination, which complicate purification processes and reduce recovery rates. Rhodium refining is limited by its extremely high melting point and resistance to chemical reactions, making extraction from ore more energy-intensive and costly. Both metals suffer from market volatility and scarcity, impacting supply chain stability and increasing refining operational risks.
Final Recommendation: Palladium vs Rhodium
Palladium and rhodium both hold significant value in refining due to their catalytic properties and market demand, with palladium typically favored for its lower price volatility and broader industrial applications, including automotive catalytic converters. Rhodium is highly prized for its superior resistance to corrosion and extreme heat, making it especially critical in high-performance refining processes but often comes at a substantially higher cost. For refining operations seeking cost-efficiency with stable market demand, palladium is generally recommended, whereas rhodium is optimal when performance and durability justify the premium investment.
Infographic: Palladium vs Rhodium for Refining
 azmater.com
azmater.com