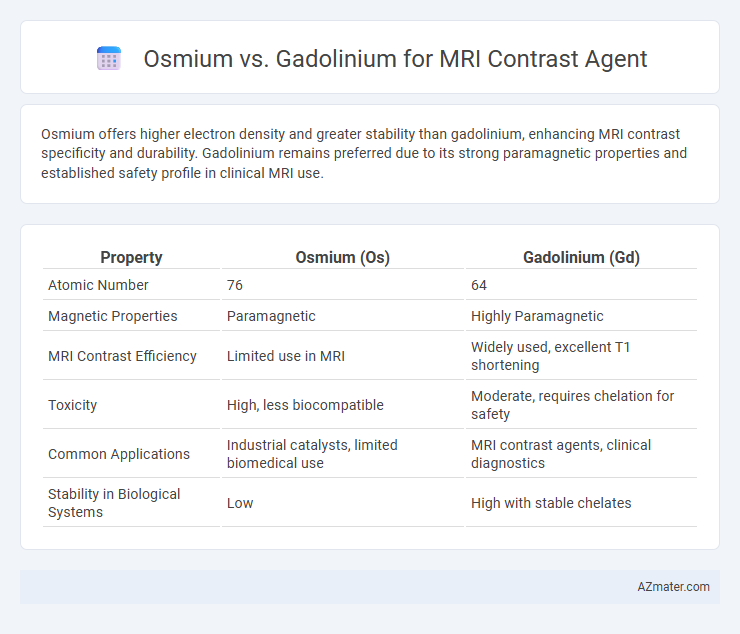
Osmium offers higher electron density and greater stability than gadolinium, enhancing MRI contrast specificity and durability. Gadolinium remains preferred due to its strong paramagnetic properties and established safety profile in clinical MRI use.
Table of Comparison
| Property | Osmium (Os) | Gadolinium (Gd) |
|---|---|---|
| Atomic Number | 76 | 64 |
| Magnetic Properties | Paramagnetic | Highly Paramagnetic |
| MRI Contrast Efficiency | Limited use in MRI | Widely used, excellent T1 shortening |
| Toxicity | High, less biocompatible | Moderate, requires chelation for safety |
| Common Applications | Industrial catalysts, limited biomedical use | MRI contrast agents, clinical diagnostics |
| Stability in Biological Systems | Low | High with stable chelates |
Introduction to MRI Contrast Agents
MRI contrast agents enhance image clarity by altering the magnetic properties of nearby water protons, improving tissue differentiation. Gadolinium-based agents are commonly used due to their strong paramagnetic properties, which shorten T1 relaxation times and provide high signal intensity. Osmium, with its unique electronic configuration and potential biocompatibility, is being explored as an alternative contrast agent to offer improved contrast efficiency and reduced toxicity.
Overview of Osmium and Gadolinium
Osmium and gadolinium are transition metals with distinct properties influencing their use as MRI contrast agents; gadolinium, a lanthanide with strong paramagnetic characteristics, is widely utilized due to its ability to shorten T1 relaxation times and enhance image contrast. Osmium, a dense platinum-group metal with less explored paramagnetic properties, presents potential alternatives for MRI contrast but lacks the extensive biocompatibility and clinical approval that gadolinium holds. Gadolinium's established safety profiles and effective magnetic behavior make it the preferred agent in current MRI practices, while osmium remains primarily experimental in this context.
Chemical Properties and Structures
Osmium, a dense transition metal with a high atomic number (76), exhibits a stable +4 to +8 oxidation state range and forms robust complexes often utilized for their durability and electron-rich properties in imaging agents. Gadolinium, a lanthanide element with atomic number 64, typically exists in the +3 oxidation state and features a half-filled 4f electron shell, granting it strong paramagnetic characteristics ideal for enhancing MRI contrast. The ionic radius of Gadolinium(III) (~93.8 pm) allows it to form stable chelates with ligands such as DTPA, while Osmium's complex structures are less commonly employed due to potential toxicity and limited paramagnetic efficiency compared to Gadolinium-based agents.
Mechanisms of MRI Contrast Enhancement
Osmium and Gadolinium influence MRI contrast through different mechanisms linked to their electronic and magnetic properties. Gadolinium, a paramagnetic lanthanide with seven unpaired electrons, enhances contrast by shortening T1 relaxation times, increasing signal intensity in T1-weighted images. Osmium, a transition metal with variable oxidation states, primarily affects T2 relaxation through susceptibility effects, causing signal dephasing and darkening regions in T2-weighted MRI scans.
Efficacy: Osmium vs Gadolinium
Osmium demonstrates promising potential as an MRI contrast agent due to its strong paramagnetic properties, which can enhance image clarity and tissue differentiation more effectively than gadolinium in certain applications. Gadolinium-based agents, widely used in clinical MRI, offer proven efficacy with high relaxation rates and established safety profiles but pose risks of nephrogenic systemic fibrosis in patients with impaired kidney function. Comparative studies indicate that osmium complexes may provide improved signal intensity and longer retention times, suggesting a viable alternative for contrast enhancement in diagnostic imaging.
Safety and Toxicity Profiles
Osmium and gadolinium are both used in MRI contrast agents, but gadolinium-based agents are more common due to their established safety and efficacy profiles. Gadolinium chelates have a well-documented toxicity profile, though concerns such as nephrogenic systemic fibrosis (NSF) in patients with renal impairment necessitate careful dosing and patient screening. Osmium compounds are less studied in clinical settings, and their potential toxicity is not fully characterized, limiting their current use despite some promising magnetic properties.
Pharmacokinetics and Biodistribution
Osmium-based MRI contrast agents exhibit slower clearance rates and prolonged blood circulation times compared to gadolinium agents, enhancing contrast duration in imaging. Gadolinium complexes demonstrate rapid renal excretion with primary accumulation in kidneys and liver, whereas osmium compounds tend to deposit in the liver, spleen, and reticuloendothelial system due to their higher molecular weight and lipophilicity. These pharmacokinetic properties influence biodistribution patterns, impacting the choice of contrast agent for specific diagnostic applications based on tissue targeting and safety profiles.
Clinical Applications and Use Cases
Osmium and gadolinium serve distinct roles in MRI contrast, with gadolinium widely established due to its high paramagnetic properties enhancing T1-weighted imaging for clearer visualization of vascular and tissue abnormalities. Osmium's clinical applications remain limited, primarily explored in experimental settings for its potential to improve contrast in specific imaging sequences without inducing nephrogenic systemic fibrosis. Gadolinium-based agents dominate MRI contrast use in neuroimaging, cardiovascular assessments, and tumor detection owing to their proven safety profiles and effective enhancement capabilities.
Cost and Availability Considerations
Osmium is significantly less common than gadolinium, resulting in higher costs and limited availability for use as an MRI contrast agent. Gadolinium, widely utilized in medical imaging, benefits from established supply chains and more cost-effective production. The abundant accessibility of gadolinium makes it the preferred choice over osmium for routine MRI diagnostic procedures.
Future Perspectives and Research Directions
Osmium and gadolinium exhibit distinct chemical properties influencing their effectiveness as MRI contrast agents, with gadolinium currently favored due to its strong paramagnetic characteristics and established safety profiles. Future research aims to enhance osmium-based agents by improving biocompatibility and targeting capabilities, potentially offering superior contrast through its unique electronic configuration. Advancements in nanotechnology and ligand design are critical for overcoming toxicity challenges and optimizing molecular stability in both elements, driving innovation in next-generation MRI diagnostics.
Infographic: Osmium vs Gadolinium for MRI Contrast Agent
 azmater.com
azmater.com