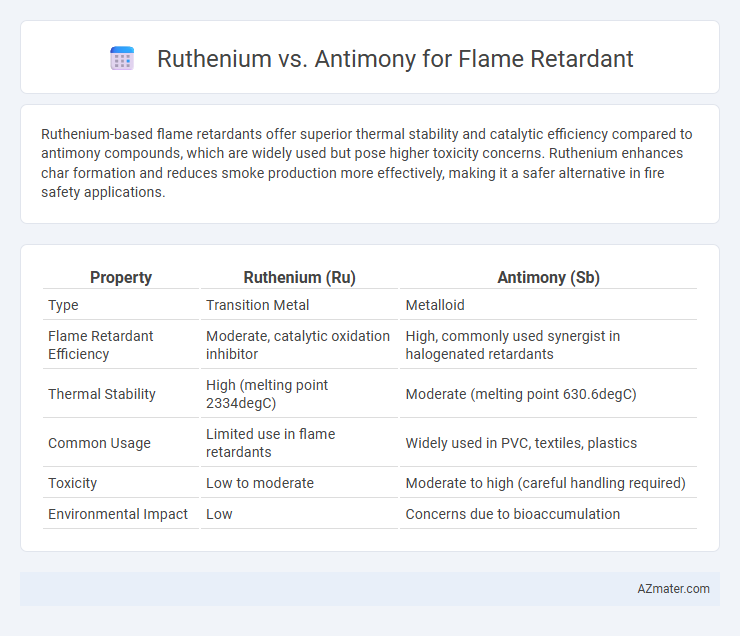
Ruthenium-based flame retardants offer superior thermal stability and catalytic efficiency compared to antimony compounds, which are widely used but pose higher toxicity concerns. Ruthenium enhances char formation and reduces smoke production more effectively, making it a safer alternative in fire safety applications.
Table of Comparison
| Property | Ruthenium (Ru) | Antimony (Sb) |
|---|---|---|
| Type | Transition Metal | Metalloid |
| Flame Retardant Efficiency | Moderate, catalytic oxidation inhibitor | High, commonly used synergist in halogenated retardants |
| Thermal Stability | High (melting point 2334degC) | Moderate (melting point 630.6degC) |
| Common Usage | Limited use in flame retardants | Widely used in PVC, textiles, plastics |
| Toxicity | Low to moderate | Moderate to high (careful handling required) |
| Environmental Impact | Low | Concerns due to bioaccumulation |
Introduction to Flame Retardants
Ruthenium and Antimony are elements utilized in flame retardant applications, but Antimony compounds, particularly Antimony trioxide, are widely recognized for their synergy with halogenated flame retardants to enhance fire resistance. Ruthenium, a rare transition metal, is less common in flame retardant formulations but shows potential in advanced materials due to its catalytic properties. Understanding the chemical behavior and thermal stability of Antimony trioxide versus emerging Ruthenium-based compounds is key for developing effective and environmentally friendly flame retardants.
Overview of Ruthenium as a Flame Retardant
Ruthenium, a transition metal with high thermal stability, is increasingly explored as a flame retardant due to its ability to catalyze the formation of char layers that inhibit combustion. Compared to traditional flame retardants like antimony trioxide, ruthenium complexes offer enhanced catalytic efficiency and reduced toxicity, making them suitable for advanced polymer applications. Its synergistic interaction with other flame retardant additives improves overall fire resistance and smoke suppression in various materials.
Overview of Antimony as a Flame Retardant
Antimony compounds, primarily antimony trioxide, serve as effective flame retardants by enhancing the performance of halogenated materials through synergistic reactions that promote char formation and inhibit flame propagation. This additive is widely used in plastics, textiles, and coatings due to its high thermal stability and ability to increase material resistance to ignition and flame spread. Compared to ruthenium, antimony is more cost-efficient and industrially established for flame retardant applications, though environmental and health considerations are driving research into alternative solutions.
Chemical Properties: Ruthenium vs Antimony
Ruthenium exhibits exceptional thermal stability and catalytic properties that enhance flame retardant efficiency by promoting char formation and reducing combustion gases. Antimony, commonly used as antimony trioxide, acts as a synergist by releasing volatile antimony oxides that inhibit flame propagation through radical scavenging in the gas phase. While ruthenium offers superior oxidative resistance and lower toxicity, antimony compounds are more cost-effective and widely implemented in commercial flame retardant systems.
Flame Retardant Mechanisms: Ruthenium and Antimony Compared
Ruthenium enhances flame retardancy primarily through catalytic charring and oxygen scavenging, promoting the formation of a stable carbonaceous layer that inhibits combustion. Antimony functions mainly as a synergist with halogen-based retardants by forming antimony halides that interfere with radical reactions in the gas phase, effectively quenching flame propagation. Compared to antimony's gas-phase flame inhibition, ruthenium's solid-phase char formation mechanism offers a distinct pathway for flame retardant performance in polymer matrices.
Performance and Efficiency in Fire Suppression
Ruthenium-based flame retardants exhibit superior catalytic efficiency in promoting char formation and radical-trapping, enhancing fire suppression performance compared to antimony compounds. Antimony oxides act mainly as synergists with halogenated flame retardants, providing moderate smoke suppression but lower thermal stability and longer reaction times than ruthenium catalysts. The higher thermal stability and faster catalytic action of ruthenium contribute to improved flame retardant efficiency and reduced toxicity during combustion.
Environmental Impact and Toxicity Assessment
Ruthenium-based flame retardants exhibit low toxicity and minimal environmental persistence, making them a safer alternative compared to traditional antimony compounds. Antimony, widely used in flame retardants, poses significant environmental risks due to its bioaccumulation potential and toxic effects on aquatic ecosystems. Comprehensive toxicity assessments highlight ruthenium's favorable environmental profile, emphasizing reduced ecological hazards and lower human health risks in flame retardant applications.
Cost Analysis: Ruthenium vs Antimony
Ruthenium offers advanced flame retardant properties but at a significantly higher cost due to its rarity and complex extraction process, making it less economically viable for large-scale use compared to antimony. Antimony, widely used in flame retardants like antimony trioxide, provides a cost-effective balance of performance and affordability, benefiting from established supply chains and lower raw material prices. When evaluating total cost of ownership, antimony remains the preferred choice for mainstream flame retardant applications, while ruthenium is typically reserved for specialized, high-performance scenarios where cost is less restrictive.
Industrial and Application Suitability
Ruthenium is rarely used as a flame retardant due to its high cost and primarily catalytic applications, making it unsuitable for large-scale industrial flame retardant solutions. Antimony, specifically in the form of antimony trioxide (Sb2O3), is widely employed in the plastics and electronics industries for its effective synergy with halogenated flame retardants, enhancing fire resistance in polymers such as polypropylene and ABS. Industrially, antimony compounds offer superior cost-efficiency and scalability, making them the preferred choice for flame retardant formulations in manufacturing processes.
Future Trends and Innovations in Flame Retardant Technologies
Ruthenium-based compounds are emerging in flame retardant technologies due to their catalytic efficiency in reducing toxic smoke and enhancing char formation. Antimony trioxide has long been used as a synergist in halogenated flame retardants, but concerns about its toxicity and environmental impact are driving research toward safer alternatives like ruthenium complexes. Future trends focus on eco-friendly, high-performance flame retardants leveraging ruthenium's unique chemical properties to meet stricter fire safety regulations and sustainability goals.
Infographic: Ruthenium vs Antimony for Flame Retardant
 azmater.com
azmater.com