Chromium offers superior corrosion resistance and hardness compared to zinc in electroplating applications. Zinc provides excellent sacrificial protection and cost-effectiveness, making it suitable for general rust prevention.
Table of Comparison
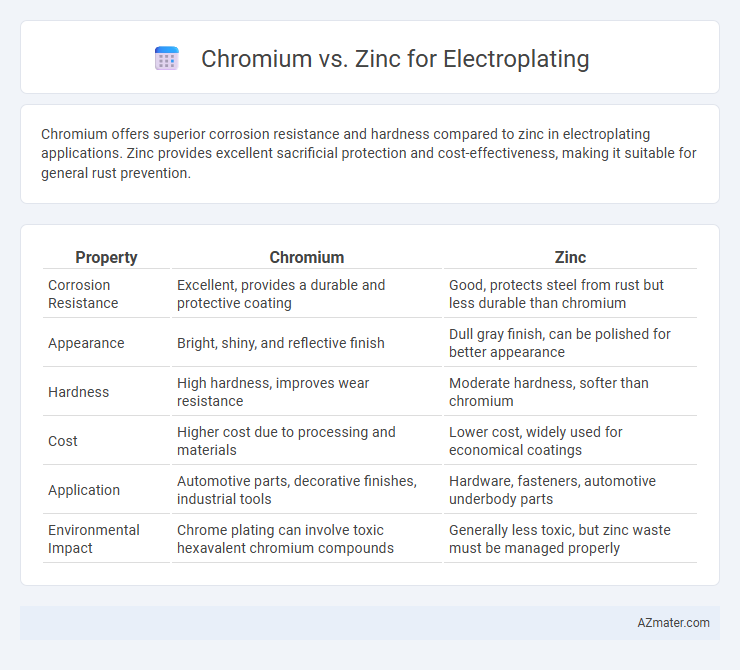
| Property | Chromium | Zinc |
|---|---|---|
| Corrosion Resistance | Excellent, provides a durable and protective coating | Good, protects steel from rust but less durable than chromium |
| Appearance | Bright, shiny, and reflective finish | Dull gray finish, can be polished for better appearance |
| Hardness | High hardness, improves wear resistance | Moderate hardness, softer than chromium |
| Cost | Higher cost due to processing and materials | Lower cost, widely used for economical coatings |
| Application | Automotive parts, decorative finishes, industrial tools | Hardware, fasteners, automotive underbody parts |
| Environmental Impact | Chrome plating can involve toxic hexavalent chromium compounds | Generally less toxic, but zinc waste must be managed properly |
Introduction to Chromium and Zinc Electroplating
Chromium electroplating provides a hard, corrosion-resistant, and aesthetically shiny surface commonly used in automotive, industrial, and decorative applications. Zinc electroplating primarily offers corrosion protection for steel components through galvanic action and is widely utilized in automotive, construction, and hardware industries. Both processes involve electrochemical deposition, but chromium plating is favored for durability and appearance, while zinc plating is preferred for cost-effective rust prevention.
Chemical Properties: Chromium vs Zinc
Chromium exhibits high corrosion resistance due to its ability to form a stable, adherent oxide layer, making it ideal for protective electroplating. Zinc offers sacrificial protection by oxidizing preferentially, effectively preventing rust on steel surfaces. The hardness and wear resistance of chromium surpass zinc, while zinc's lower toxicity and cost provide a more economical plating option for non-critical applications.
Electroplating Process Overview
Chromium electroplating involves applying a thin, hard chromium layer that provides corrosion resistance and improves surface hardness, utilizing hexavalent or trivalent chromium solutions in a controlled acidic bath. Zinc electroplating deposits a zinc layer primarily for rust prevention on steel, using alkaline or acid zinc plating baths with additives to ensure uniform coating and adhesion. Both processes require precise control of current density, temperature, and bath chemistry to achieve optimal coating thickness and quality.
Corrosion Resistance Comparison
Chromium provides superior corrosion resistance in electroplating due to its ability to form a dense, hard, and passivating oxide layer that effectively protects the base metal from environmental degradation. Zinc offers moderate corrosion resistance by creating a sacrificial coating that corrodes preferentially, protecting the underlying metal but lacking the durability of chromium. The choice between chromium and zinc depends on application requirements, with chromium preferred for high-wear and high-corrosion environments, while zinc is favored for cost-effective, moderate protection.
Surface Finish and Appearance
Chromium electroplating produces a bright, reflective, and highly durable surface finish, often used for decorative and corrosion-resistant coatings on automotive parts and appliances. Zinc plating provides a matte to shiny finish mainly valued for its corrosion protection rather than aesthetic appeal, commonly applied to fasteners and hardware. Chromium's hardness and resistance to tarnishing result in a longer-lasting, mirror-like appearance compared to zinc's softer, more utilitarian coating.
Cost Analysis: Chromium vs Zinc Plating
Chromium plating typically incurs higher costs due to expensive raw materials and energy-intensive processes, whereas zinc plating offers a more economical alternative with lower material expenses and simpler application methods. The cost-efficiency of zinc plating makes it preferable for large-scale industrial use, especially when corrosion resistance requirements are moderate. Chromium plating remains favored for applications demanding superior hardness and aesthetic appeal, despite its premium pricing.
Environmental and Safety Considerations
Chromium electroplating involves toxic hexavalent chromium compounds, posing significant environmental hazards and requiring stringent safety protocols to prevent carcinogenic exposure and water contamination. Zinc electroplating uses less toxic materials and produces fewer hazardous byproducts, making it a safer and more environmentally friendly option for corrosion protection. Waste management practices for chromium plating are more complex and costly due to the need for specialized treatment of chromate-containing effluents.
Common Industrial Applications
Chromium and zinc are widely used in electroplating for corrosion resistance and aesthetic enhancement, with chromium commonly applied in automotive parts, aerospace, and decorative finishes due to its hardness and high corrosion resistance. Zinc electroplating is primarily utilized in the construction, automotive, and electrical industries to provide sacrificial protection against rust on steel and iron components. Both metals ensure durability and improved lifespan of industrial equipment, with chromium favored for wear resistance and zinc for economical corrosion prevention.
Durability and Maintenance Requirements
Chromium electroplating offers superior durability with exceptional hardness and corrosion resistance, making it ideal for high-wear applications. Zinc plating provides moderate protection against corrosion but requires periodic maintenance such as clear coating or passivation to prevent rusting. The maintenance demands for zinc-plated surfaces are generally higher than chromium, which benefits from long-lasting, low-maintenance finishes in industrial environments.
Choosing the Right Metal for Electroplating Needs
Choosing between chromium and zinc for electroplating depends on factors like corrosion resistance, aesthetic appeal, and cost-effectiveness. Chromium plating offers superior hardness, high corrosion resistance, and a bright, reflective finish ideal for automotive and decorative applications. Zinc plating provides excellent rust protection and affordability, making it suitable for industrial components exposed to harsh environments.
Infographic: Chromium vs Zinc for Electroplating
 azmater.com
azmater.com