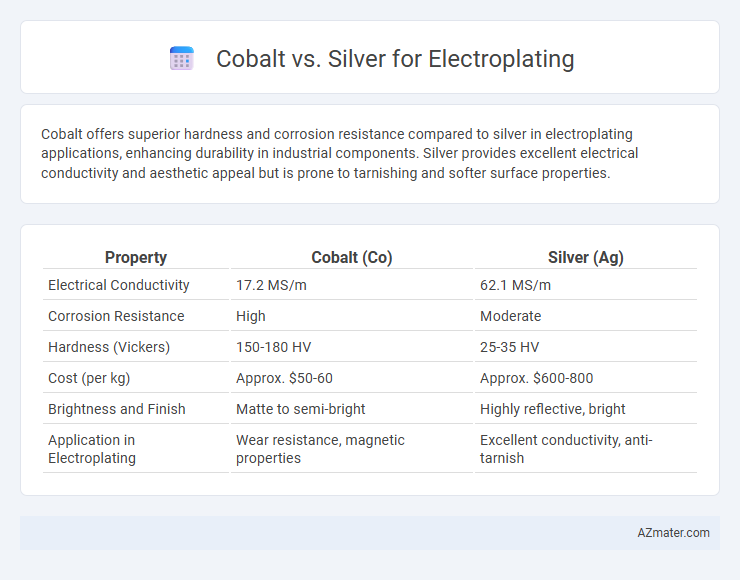
Cobalt offers superior hardness and corrosion resistance compared to silver in electroplating applications, enhancing durability in industrial components. Silver provides excellent electrical conductivity and aesthetic appeal but is prone to tarnishing and softer surface properties.
Table of Comparison
| Property | Cobalt (Co) | Silver (Ag) |
|---|---|---|
| Electrical Conductivity | 17.2 MS/m | 62.1 MS/m |
| Corrosion Resistance | High | Moderate |
| Hardness (Vickers) | 150-180 HV | 25-35 HV |
| Cost (per kg) | Approx. $50-60 | Approx. $600-800 |
| Brightness and Finish | Matte to semi-bright | Highly reflective, bright |
| Application in Electroplating | Wear resistance, magnetic properties | Excellent conductivity, anti-tarnish |
Introduction to Electroplating: Cobalt vs Silver
Electroplating involves depositing a metal coating onto a substrate to enhance corrosion resistance, conductivity, or aesthetic appeal, with cobalt and silver being prominent choices. Cobalt offers superior hardness, wear resistance, and magnetic properties, making it ideal for industrial and electronic applications. Silver excels in electrical conductivity and offers a bright, reflective finish, commonly used in decorative and electrical contacts.
Chemical Properties: Cobalt and Silver Compared
Cobalt exhibits excellent corrosion resistance and high hardness due to its hexagonal close-packed crystal structure, making it ideal for durable electroplating applications. Silver offers superior electrical conductivity and reflectivity, derived from its face-centered cubic crystal lattice, which enhances surface brightness and is preferred for decorative plating. Both metals form stable, adherent coatings, but cobalt's chemical stability under harsh conditions contrasts with silver's susceptibility to tarnishing from sulfur compounds.
Electroplating Efficiency: Cobalt vs Silver
Cobalt exhibits higher electroplating efficiency than silver due to its lower overpotential and superior deposit adhesion, resulting in smoother, more uniform coatings with less energy consumption. Silver's electroplating process often requires careful control of complexing agents to prevent rough or dendritic deposits, which can reduce efficiency and increase material waste. Overall, cobalt's electrochemical properties enable faster deposition rates and enhanced layer durability, making it a more efficient choice for certain industrial electroplating applications.
Surface Finish and Appearance Differences
Cobalt electroplating produces a harder, more durable surface with a slightly bluish tint, enhancing scratch resistance and wear performance, while silver plating offers a bright, highly reflective finish with superior conductivity but is softer and more prone to tarnishing. Silver's lustrous appearance is ideal for decorative and electrical applications requiring high reflectivity, whereas cobalt provides a matte to semi-bright finish suitable for industrial uses demanding corrosion resistance. The difference in microstructure and plating thickness significantly impacts the overall aesthetic and longevity of the coated item.
Durability and Corrosion Resistance
Cobalt electroplating offers superior durability and enhanced corrosion resistance compared to silver, making it ideal for high-wear applications. Silver provides excellent electrical conductivity and a bright finish but is more susceptible to tarnishing and corrosion over time. Cobalt's hardness and resistance to oxidation ensure longer-lasting coatings in harsh industrial environments.
Cost Analysis: Cobalt vs Silver Plating
Cobalt plating generally offers a more cost-effective solution compared to silver plating due to its lower raw material expenses and longer-lasting durability, reducing the need for frequent reapplication. Silver plating, while more expensive upfront because of precious metal prices, provides superior conductivity and corrosion resistance, which may justify higher costs in specialized applications. Cost analysis must consider factors such as material price volatility, plating thickness requirements, and lifecycle maintenance expenses to determine the optimal choice between cobalt and silver plating.
Environmental Impact and Safety Considerations
Cobalt electroplating poses significant environmental challenges due to toxic waste generation and potential groundwater contamination, while silver electroplating presents lower toxicity but raises concerns about silver ion release into aquatic ecosystems. Handling cobalt requires strict safety protocols because of its carcinogenic and allergenic properties, whereas silver necessitates caution to prevent skin and respiratory irritation. Waste treatment for cobalt plating is more complex and costly, making silver a comparatively safer and environmentally preferable option for electroplating applications.
Common Applications of Cobalt and Silver Electroplating
Cobalt electroplating is commonly used in aerospace and automotive industries due to its excellent wear resistance, corrosion protection, and ability to maintain hardness at high temperatures. Silver electroplating is favored for electrical contacts, jewelry, and decorative items because of its superior electrical conductivity, aesthetic appeal, and tarnish resistance. Both metals enhance surface properties, but cobalt is preferred for durability and silver for conductivity and appearance.
Technical Challenges and Limitations
Cobalt electroplating faces technical challenges such as maintaining uniform thickness and controlling internal stress to prevent cracking, which can limit its use in precision applications compared to silver. Silver electroplating offers excellent conductivity and corrosion resistance but suffers from susceptibility to tarnishing and requires rigorous surface preparation to ensure adhesion. Both metals demand careful control of bath chemistry and operating conditions to overcome limitations related to wear resistance and environmental impact during plating.
Conclusion: Choosing Between Cobalt and Silver for Electroplating
Cobalt offers excellent hardness, corrosion resistance, and cost-efficiency, making it ideal for industrial applications requiring durability and wear resistance. Silver provides superior electrical conductivity and aesthetic appeal, preferred in decorative and electronics plating where appearance and electrical performance matter. The choice between cobalt and silver depends on prioritizing mechanical strength and corrosion resistance versus conductivity and visual quality.
Infographic: Cobalt vs Silver for Electroplating
 azmater.com
azmater.com