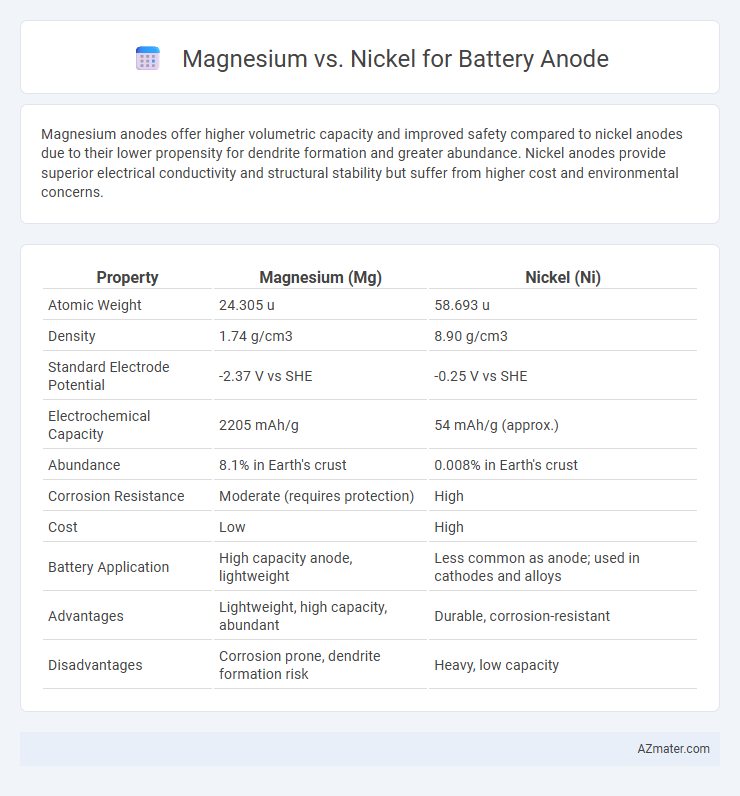
Magnesium anodes offer higher volumetric capacity and improved safety compared to nickel anodes due to their lower propensity for dendrite formation and greater abundance. Nickel anodes provide superior electrical conductivity and structural stability but suffer from higher cost and environmental concerns.
Table of Comparison
| Property | Magnesium (Mg) | Nickel (Ni) |
|---|---|---|
| Atomic Weight | 24.305 u | 58.693 u |
| Density | 1.74 g/cm3 | 8.90 g/cm3 |
| Standard Electrode Potential | -2.37 V vs SHE | -0.25 V vs SHE |
| Electrochemical Capacity | 2205 mAh/g | 54 mAh/g (approx.) |
| Abundance | 8.1% in Earth's crust | 0.008% in Earth's crust |
| Corrosion Resistance | Moderate (requires protection) | High |
| Cost | Low | High |
| Battery Application | High capacity anode, lightweight | Less common as anode; used in cathodes and alloys |
| Advantages | Lightweight, high capacity, abundant | Durable, corrosion-resistant |
| Disadvantages | Corrosion prone, dendrite formation risk | Heavy, low capacity |
Introduction: Magnesium vs Nickel as Battery Anode Materials
Magnesium and nickel serve distinct roles as battery anode materials with unique electrochemical properties. Magnesium offers high volumetric capacity and abundant availability, making it a promising candidate for next-generation batteries with enhanced energy density. Nickel provides excellent conductivity and corrosion resistance, widely used in hybrid and nickel-based batteries to improve cycle life and stability.
Fundamental Properties of Magnesium and Nickel
Magnesium exhibits a high volumetric capacity of approximately 3833 mAh/cm3, significantly surpassing nickel's capacity near 60 mAh/g, making magnesium a promising candidate for high-energy anodes in batteries. Magnesium's divalent ion (Mg2+) enables more efficient charge transfer compared to nickel's monovalent ion behavior, resulting in potential improvements in battery performance and longevity. However, nickel provides excellent structural stability and corrosion resistance, crucial for maintaining anode integrity during repeated charge-discharge cycles.
Electrochemical Performance Comparison
Magnesium anodes exhibit higher volumetric capacity (3,833 mAh/cm3) compared to nickel's lower specific capacity and often face slower diffusion kinetics affecting charge rates. Nickel anodes, while possessing excellent electrical conductivity and stable cycling performance, generally provide lower energy density than magnesium counterparts. Electrochemical performance analysis reveals magnesium's potential for high-capacity, cost-effective batteries, though nickel's enhanced stability offers advantages in long-term cycle life.
Capacity and Energy Density Analysis
Magnesium anodes offer a theoretical capacity of approximately 2205 mAh/g, significantly higher than nickel's capacity of around 55 mAh/g, making magnesium more suitable for high-capacity battery applications. Energy density analysis shows magnesium-based batteries can achieve up to 3833 Wh/kg, surpassing nickel-based counterparts, which typically deliver lower energy densities due to nickel's limited capacity and higher atomic weight. The high volumetric and gravimetric energy densities of magnesium anodes enable longer-lasting and more efficient energy storage solutions compared to nickel anode batteries.
Cycle Life and Stability Evaluation
Magnesium anodes offer higher volumetric capacity and improved dendrite suppression compared to nickel, leading to enhanced cycle life in battery applications. However, nickel anodes provide superior electrochemical stability under varied charge-discharge conditions, contributing to consistent performance over long-term cycling. Stability evaluations reveal magnesium's susceptibility to surface passivation, while nickel maintains a robust interface, making nickel favorable for applications demanding extended cycle durability.
Cost and Resource Availability
Magnesium anodes offer significant cost advantages over nickel due to magnesium's abundant crustal abundance at approximately 2.3% compared to nickel's 0.008%, translating to lower raw material expenses and greater long-term availability. Nickel production involves energy-intensive refining processes and geopolitical supply risks, which can drive price volatility, whereas magnesium extraction from seawater or minerals remains relatively stable and cost-effective. The widespread availability and scalability of magnesium resources position it as a more economically sustainable choice for battery anodes in large-scale energy storage applications.
Safety and Thermal Stability Considerations
Magnesium anodes exhibit superior safety and thermal stability compared to nickel due to their lower reactivity and reduced risk of dendrite formation, which minimizes short-circuit hazards. Nickel anodes, while offering high energy density, are prone to thermal runaway and overheating under stress, leading to potential fire risks. Thermal stability of magnesium enables more reliable battery performance in high-temperature applications, enhancing overall safety standards.
Environmental Impact and Sustainability
Magnesium anodes offer a more sustainable alternative to nickel due to their greater abundance in the Earth's crust and lower environmental extraction impact, resulting in reduced ecological footprint. Nickel mining and processing generate significant carbon emissions and toxic waste, posing challenges for long-term environmental sustainability in battery production. Using magnesium in battery anodes can enhance recyclability and lower reliance on conflict-prone supply chains, contributing to greener energy storage solutions.
Current Commercial and Research Applications
Magnesium anodes offer higher volumetric capacity and abundant availability, making them promising for next-generation batteries, particularly in research targeting safer and cost-effective energy storage solutions. Nickel anodes, extensively used in commercial nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries, provide reliable cycling stability and high power density suitable for portable electronics and hybrid vehicles. Current research explores magnesium anodes to overcome dendrite formation challenges while improving conductivity, whereas nickel remains dominant in established commercial applications due to mature manufacturing processes and proven performance.
Future Prospects and Challenges
Magnesium anodes offer higher volumetric capacity and abundant resources, making them promising for next-generation batteries with increased energy density and safety. Nickel anodes provide excellent conductivity and stability but face challenges in scalability and cost-effectiveness for large-scale energy storage. Future research focuses on improving magnesium ion mobility and overcoming nickel's material limitations to enhance battery performance and lifespan.
Infographic: Magnesium vs Nickel for Battery Anode
 azmater.com
azmater.com