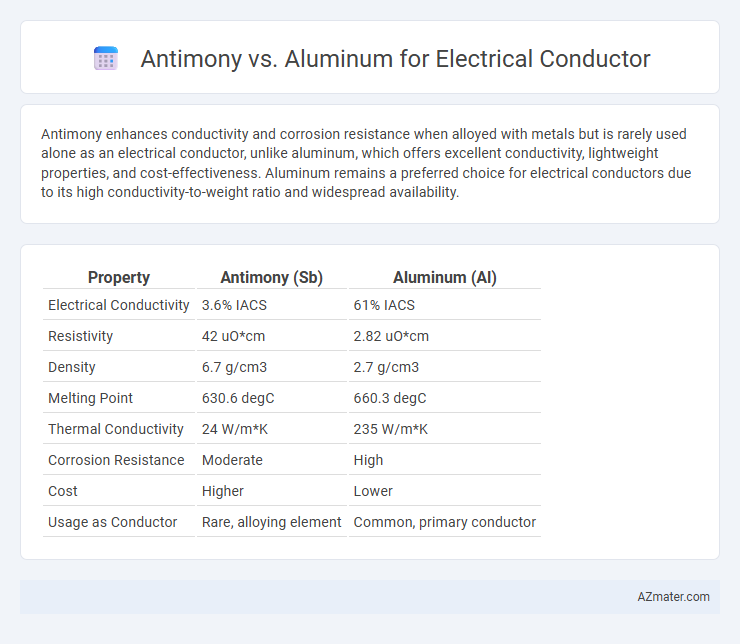
Antimony enhances conductivity and corrosion resistance when alloyed with metals but is rarely used alone as an electrical conductor, unlike aluminum, which offers excellent conductivity, lightweight properties, and cost-effectiveness. Aluminum remains a preferred choice for electrical conductors due to its high conductivity-to-weight ratio and widespread availability.
Table of Comparison
| Property | Antimony (Sb) | Aluminum (Al) |
|---|---|---|
| Electrical Conductivity | 3.6% IACS | 61% IACS |
| Resistivity | 42 uO*cm | 2.82 uO*cm |
| Density | 6.7 g/cm3 | 2.7 g/cm3 |
| Melting Point | 630.6 degC | 660.3 degC |
| Thermal Conductivity | 24 W/m*K | 235 W/m*K |
| Corrosion Resistance | Moderate | High |
| Cost | Higher | Lower |
| Usage as Conductor | Rare, alloying element | Common, primary conductor |
Introduction: Antimony vs Aluminum as Electrical Conductors
Antimony and aluminum exhibit distinct electrical conductivity characteristics crucial for conductor applications. Aluminum offers a high conductivity-to-weight ratio, making it a popular choice for overhead power lines due to its lightness and cost-effectiveness. Antimony, typically used as an alloying element, improves mechanical strength and corrosion resistance in electrical conductors but is not used alone as a primary conductor material.
Electrical Conductivity Comparison
Aluminum exhibits significantly higher electrical conductivity, approximately 61% of copper's conductivity, making it a preferred material for electrical conductors in power transmission. Antimony, by contrast, has much lower electrical conductivity and is primarily used as an alloying element to enhance mechanical properties rather than as a conductor itself. The superior conductivity of aluminum combined with its lightweight and cost-effectiveness ensures its widespread application over antimony in electrical wiring and cables.
Material Properties and Composition
Antimony is primarily used as an alloying element to improve the mechanical strength and hardness of electrical conductors, notably in lead-based cables, whereas aluminum serves as a lightweight and highly conductive metal suitable for power transmission lines. Aluminum's conductivity is approximately 61% that of copper, combined with its excellent corrosion resistance and low density (2.7 g/cm3) making it ideal for large-scale electrical applications. In contrast, antimony's role is focused on enhancing material properties such as wear resistance and thermal stability rather than serving as a primary conductor due to its poor electrical conductivity.
Thermal Performance in Electrical Applications
Antimony enhances the thermal stability of electrical conductors by improving the heat resistance of aluminum alloys, making them suitable for high-temperature electrical applications. Aluminum, known for its excellent thermal conductivity (~237 W/m*K), efficiently dissipates heat, reducing the risk of overheating in conductors. Combining antimony with aluminum alloys balances thermal conductivity and mechanical strength, optimizing performance for electrical wiring and components exposed to elevated temperatures.
Corrosion Resistance and Longevity
Antimony enhances aluminum's corrosion resistance when used as an alloying element, reducing susceptibility to oxidation and extending the conductor's lifespan in harsh environments. Pure aluminum offers good conductivity but corrodes more quickly, especially in marine or industrial atmospheres. Aluminum-antimony alloys maintain electrical performance while significantly improving durability and longevity for electrical conductors.
Mechanical Strength and Flexibility
Antimony enhances the mechanical strength of copper alloys, improving durability without significantly compromising electrical conductivity, making it suitable for applications requiring robust conductors. Aluminum is lightweight and offers excellent flexibility, which facilitates easier installation and bending in electrical wiring systems, although it has lower mechanical strength compared to antimony-alloyed conductors. The choice between antimony-enhanced conductors and aluminum depends on balancing strength requirements with flexibility and weight constraints in electrical infrastructure.
Cost Analysis and Market Availability
Antimony, primarily used as an alloying element, has limited application as a direct electrical conductor compared to aluminum, which is widely recognized for its excellent conductivity and cost-effectiveness. Aluminum offers a lower cost per kilogram and greater market availability, making it the preferred choice for large-scale electrical wiring and power line applications. The abundance of aluminum in global markets ensures stable pricing, whereas antimony's scarcity and specialized use contribute to higher costs and limited supply for conductive purposes.
Environmental Impact and Sustainability
Antimony, often used as an alloying element in lead-acid batteries and flame retardants, poses significant environmental concerns due to its toxicity and limited recycling infrastructure, leading to soil and water contamination risks. Aluminum, with its abundant availability, excellent recyclability, and lower toxicity profile, offers a more sustainable choice for electrical conductors despite the high energy consumption during initial smelting. Choosing aluminum over antimony can reduce environmental impact and support sustainable electrical infrastructure development.
Common Applications in the Electrical Industry
Antimony alloys enhance lead-acid battery grids and cable sheathing due to their improved hardness and corrosion resistance, contributing to reliable electrical performance. Aluminum remains a preferred material for overhead power lines and electrical wiring because of its excellent conductivity-to-weight ratio and cost-effectiveness. The combination of aluminum's lightweight and antimony's strengthening properties addresses specific industry needs for durability and efficiency in electrical conductors.
Conclusion: Selecting the Optimal Electrical Conductor
Antimony and aluminum differ significantly in electrical conductivity, with aluminum offering superior conductivity (approximately 37.7 million Siemens per meter) compared to antimony, which is primarily used as an alloying element rather than a conductor. Aluminum's low density and high conductivity make it the optimal choice for overhead power lines and electrical wiring, balancing cost efficiency and performance. Antimony's role is more specialized in improving properties of alloys rather than serving as a primary electrical conductor.
Infographic: Antimony vs Aluminum for Electrical Conductor
 azmater.com
azmater.com