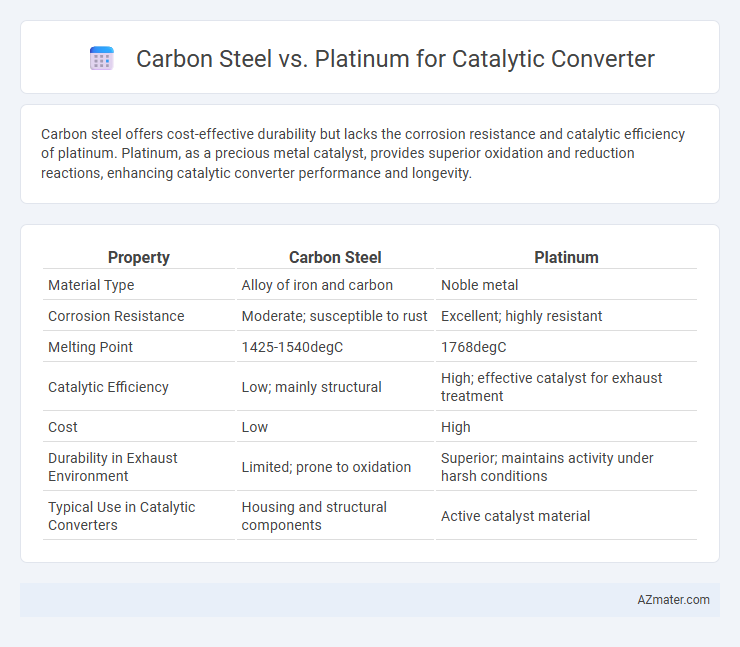
Carbon steel offers cost-effective durability but lacks the corrosion resistance and catalytic efficiency of platinum. Platinum, as a precious metal catalyst, provides superior oxidation and reduction reactions, enhancing catalytic converter performance and longevity.
Table of Comparison
| Property | Carbon Steel | Platinum |
|---|---|---|
| Material Type | Alloy of iron and carbon | Noble metal |
| Corrosion Resistance | Moderate; susceptible to rust | Excellent; highly resistant |
| Melting Point | 1425-1540degC | 1768degC |
| Catalytic Efficiency | Low; mainly structural | High; effective catalyst for exhaust treatment |
| Cost | Low | High |
| Durability in Exhaust Environment | Limited; prone to oxidation | Superior; maintains activity under harsh conditions |
| Typical Use in Catalytic Converters | Housing and structural components | Active catalyst material |
Introduction to Catalytic Converter Materials
Catalytic converters typically use metals such as platinum due to their exceptional catalytic properties and resistance to high temperatures and corrosion. Carbon steel, although cost-effective and strong, lacks the durability and catalytic efficiency required for effective exhaust emission control. Platinum offers superior oxidation and reduction reactions crucial for converting harmful gases into less toxic emissions, making it the preferred material in catalytic converter manufacturing.
Overview of Carbon Steel and Platinum
Carbon steel is an iron alloy known for its strength, affordability, and ease of fabrication, making it a common choice for catalytic converter substrates and housing components due to its durability and corrosion resistance. Platinum, a precious metal, serves as a highly effective catalyst in catalytic converters, offering exceptional resistance to oxidation and high catalytic activity for converting harmful exhaust gases like carbon monoxide, hydrocarbons, and nitrogen oxides into less harmful emissions. While carbon steel provides structural support, platinum acts as the active catalytic agent, enabling efficient emissions control in automotive exhaust systems.
Chemical Properties: Carbon Steel vs Platinum
Carbon steel, primarily composed of iron and carbon, exhibits moderate corrosion resistance but is prone to oxidation and rust under high-temperature exhaust conditions. Platinum, a noble metal with exceptional chemical stability, resists oxidation and maintains catalytic activity even at elevated temperatures, enabling efficient conversion of harmful emissions. The superior chemical inertness and high catalytic efficiency of platinum make it a preferred material for catalytic converters compared to carbon steel.
Performance in Catalytic Reactions
Platinum outperforms carbon steel in catalytic converters due to its superior catalytic activity and resistance to oxidation at high temperatures, enhancing the conversion of harmful gases like CO, NOx, and hydrocarbons. Carbon steel lacks the necessary surface properties and durability for efficient catalytic reactions, resulting in lower conversion efficiency and shorter lifespan. The high catalytic efficiency of platinum ensures optimal emission control and compliance with stringent environmental regulations.
Durability and Corrosion Resistance
Platinum exhibits superior durability and corrosion resistance compared to carbon steel when used in catalytic converters, maintaining its catalytic properties under high temperatures and harsh exhaust conditions. Carbon steel is prone to oxidation and degradation over time, especially in the presence of moisture and exhaust gases, leading to reduced lifespan. The inherent resistance of platinum to chemical wear ensures longer-term efficiency and consistent emission control.
Cost Comparison: Carbon Steel vs Platinum
Carbon steel offers a significantly lower material cost compared to platinum, making it an economical choice for catalytic converter substrates. Platinum, a precious metal with high market value fluctuations, increases the overall expense due to its scarcity and superior catalytic properties. The price differential is a key factor influencing manufacturing decisions, with platinum-based converters commanding higher retail prices but providing enhanced durability and efficiency.
Efficiency in Emission Reduction
Platinum is highly efficient in catalytic converters due to its superior ability to catalyze oxidation and reduction reactions, significantly reducing harmful emissions like carbon monoxide, hydrocarbons, and nitrogen oxides. Carbon steel, while more cost-effective, lacks the catalytic properties necessary for effective emission reduction and is generally used for structural components rather than active catalytic functions. The high catalytic efficiency of platinum makes it the preferred choice in emission control systems to meet stringent environmental regulations.
Environmental Impact and Sustainability
Carbon steel catalytic converters often contribute to higher environmental footprints due to increased emissions during production and lower recyclability compared to platinum-based converters. Platinum, a precious metal with exceptional catalytic properties, significantly reduces harmful exhaust gases and offers enhanced durability, promoting sustainability through longer service life and improved pollutant breakdown. Despite its higher cost, platinum's efficiency in emissions control and potential for recycling supports better environmental outcomes and resource conservation in automotive applications.
Industrial Applications and Market Trends
Carbon steel offers cost-effective durability and corrosion resistance for catalytic converters in heavy-duty industrial applications such as automotive exhaust systems and chemical processing. Platinum, valued for its superior catalytic efficiency and resistance to high temperatures, dominates high-performance catalytic converters despite higher costs, driving demand in automotive and environmental sectors. Market trends indicate growing adoption of platinum-group metals in catalytic converters due to strict emissions regulations, while advancements in coatings and alloys are improving carbon steel's competitiveness in industrial catalytic applications.
Future Prospects for Catalytic Converter Materials
Platinum remains the dominant catalytic converter material due to its exceptional catalytic efficiency and resistance to high temperatures, essential for meeting increasingly stringent emission standards. Future prospects indicate ongoing research into reducing platinum content by alloying with base metals like carbon steel, aiming to lower costs while maintaining performance. Advances in nano-engineered composites and alternative catalysts based on carbon steel frameworks promise a balance between durability, cost-effectiveness, and environmental compliance in next-generation catalytic converters.
Infographic: Carbon steel vs Platinum for Catalytic converter
 azmater.com
azmater.com