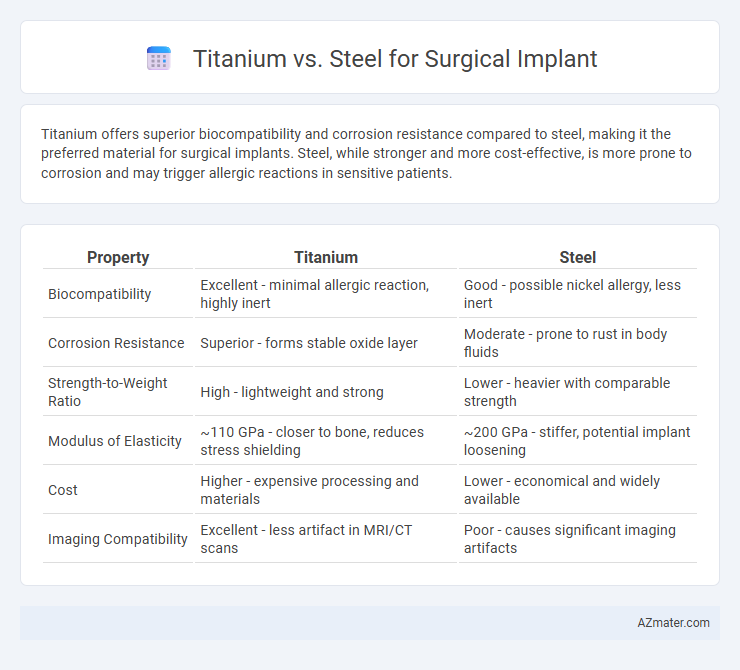
Titanium offers superior biocompatibility and corrosion resistance compared to steel, making it the preferred material for surgical implants. Steel, while stronger and more cost-effective, is more prone to corrosion and may trigger allergic reactions in sensitive patients.
Table of Comparison
| Property | Titanium | Steel |
|---|---|---|
| Biocompatibility | Excellent - minimal allergic reaction, highly inert | Good - possible nickel allergy, less inert |
| Corrosion Resistance | Superior - forms stable oxide layer | Moderate - prone to rust in body fluids |
| Strength-to-Weight Ratio | High - lightweight and strong | Lower - heavier with comparable strength |
| Modulus of Elasticity | ~110 GPa - closer to bone, reduces stress shielding | ~200 GPa - stiffer, potential implant loosening |
| Cost | Higher - expensive processing and materials | Lower - economical and widely available |
| Imaging Compatibility | Excellent - less artifact in MRI/CT scans | Poor - causes significant imaging artifacts |
Introduction: Importance of Material Choice in Surgical Implants
Material choice in surgical implants crucially affects biocompatibility, mechanical strength, and patient recovery outcomes. Titanium exhibits superior corrosion resistance and osseointegration properties compared to steel, minimizing rejection risks and enhancing implant longevity. Steel, often stainless steel, offers high tensile strength and cost-effectiveness but may pose higher risks of allergic reactions and corrosion in the body.
Composition and Properties: Titanium vs Steel
Titanium surgical implants consist primarily of titanium alloy, known for its high strength-to-weight ratio, excellent corrosion resistance, and biocompatibility, which reduces the risk of allergic reactions and promotes osseointegration. In contrast, steel implants, typically made from stainless steel containing iron, chromium, and nickel, offer greater rigidity but are heavier and more prone to corrosion within the body. Titanium's low modulus of elasticity closely matches human bone, minimizing stress shielding, whereas steel's higher modulus can lead to increased implant-related complications.
Biocompatibility: How the Body Reacts
Titanium exhibits superior biocompatibility compared to steel, as its oxide layer minimizes corrosion and reduces the risk of adverse immune reactions. The body's tissues tend to integrate more effectively with titanium implants, promoting osteointegration and reducing inflammation. Steel, particularly stainless variants, may provoke higher incidences of allergic responses and metal ion release, impacting long-term implant success.
Strength and Durability Comparison
Titanium offers superior strength-to-weight ratio compared to steel, making it highly suitable for surgical implants requiring both robustness and lightweight properties. Its exceptional corrosion resistance enhances durability in the body's biological environment, reducing the risk of implant degradation over time. Steel, while strong and cost-effective, is prone to corrosion and may require additional coatings to maintain longevity in surgical applications.
Corrosion Resistance in Biological Environments
Titanium exhibits superior corrosion resistance in biological environments compared to steel, primarily due to its stable oxide layer that protects against degradation and ion release. Stainless steel, often prone to localized corrosion such as pitting or crevice corrosion in bodily fluids, may trigger inflammatory responses and compromise implant longevity. The enhanced biocompatibility and resistance to corrosion of titanium make it the preferred material for long-term surgical implants in corrosive physiological conditions.
Weight Differences: Impact on Patient Outcomes
Titanium's density of approximately 4.5 g/cm3 makes it significantly lighter than steel, which has a density around 7.8 g/cm3, resulting in implants that reduce overall implant weight by up to 40%. This weight difference enhances patient comfort, reduces stress shielding, and promotes faster recovery by minimizing bone resorption. Lighter implants also contribute to improved mobility and decreased risk of implant-related complications in surgical applications.
Osseointegration: Bone Attachment Efficacy
Titanium exhibits superior osseointegration compared to steel, forming a strong, direct bond with bone tissue due to its biocompatible oxide layer that promotes cellular adhesion and growth. The porous surface of titanium implants enhances bone ingrowth, improving implant stability and long-term fixation. Steel, while mechanically robust, shows inferior osseointegration, often resulting in fibrous tissue encapsulation rather than direct bone bonding, which may compromise implant longevity.
Common Applications in Surgical Procedures
Titanium is widely used in surgical implants for orthopedic applications such as joint replacements, bone plates, and dental implants due to its excellent biocompatibility, corrosion resistance, and lightweight properties. Steel, particularly stainless steel alloys, is commonly employed in temporary implants like screws, plates, and intramedullary rods for fracture fixation because of its high strength and cost-effectiveness. Both materials play crucial roles in surgical procedures, with titanium preferred for permanent implants and steel often reserved for short-term or load-bearing applications.
Cost Analysis: Titanium vs Steel Implants
Titanium implants generally have higher upfront costs compared to steel implants due to expensive raw materials and specialized manufacturing processes. However, titanium's superior biocompatibility and corrosion resistance often reduce long-term expenses by minimizing implant failure rates and revision surgeries. Steel implants offer lower initial prices but may incur additional costs over time due to higher risks of allergic reactions and corrosion-related complications.
Summary: Choosing the Best Material for Surgical Implants
Titanium offers superior biocompatibility, corrosion resistance, and a high strength-to-weight ratio, making it ideal for surgical implants that require long-term durability and minimal adverse tissue reactions. Steel, particularly stainless steel, provides excellent mechanical strength and is more cost-effective but may pose higher risks of corrosion and allergic responses in some patients. Selecting between titanium and steel for surgical implants depends on factors such as patient sensitivity, implant location, and the balance between performance requirements and budget constraints.
Infographic: Titanium vs Steel for Surgical Implant
 azmater.com
azmater.com