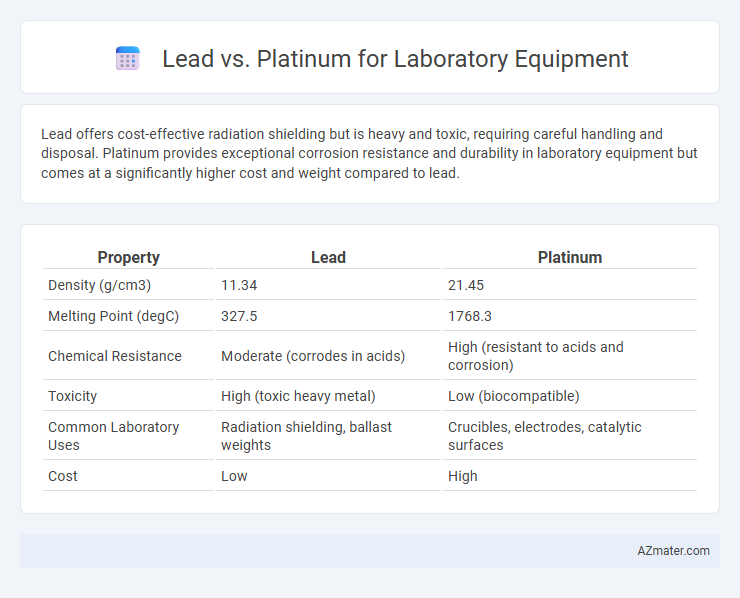
Lead offers cost-effective radiation shielding but is heavy and toxic, requiring careful handling and disposal. Platinum provides exceptional corrosion resistance and durability in laboratory equipment but comes at a significantly higher cost and weight compared to lead.
Table of Comparison
| Property | Lead | Platinum |
|---|---|---|
| Density (g/cm3) | 11.34 | 21.45 |
| Melting Point (degC) | 327.5 | 1768.3 |
| Chemical Resistance | Moderate (corrodes in acids) | High (resistant to acids and corrosion) |
| Toxicity | High (toxic heavy metal) | Low (biocompatible) |
| Common Laboratory Uses | Radiation shielding, ballast weights | Crucibles, electrodes, catalytic surfaces |
| Cost | Low | High |
Introduction to Laboratory Metals: Lead and Platinum
Lead and platinum are common metals used in laboratory equipment due to their distinct physical and chemical properties. Lead offers excellent radiation shielding and corrosion resistance, making it ideal for protective barriers and containers in radioactive environments. Platinum's high melting point, inertness, and exceptional conductivity make it suitable for electrodes, crucibles, and high-temperature applications.
Physical and Chemical Properties Comparison
Lead exhibits high density (11.34 g/cm3) and low melting point (327.5degC), with good corrosion resistance but is prone to oxidation and toxicity concerns, making it less ideal for certain lab environments. Platinum, with a density of 21.45 g/cm3 and melting point of 1768degC, offers exceptional chemical inertness and resistance to corrosion and high temperatures, making it highly suitable for laboratory equipment requiring durability and chemical stability. The superior hardness and non-reactivity of platinum surpass lead in applications demanding precise and contamination-free conditions.
Historical Uses of Lead and Platinum in Laboratories
Lead has historically been utilized in laboratories for its dense, malleable properties, serving as radiation shields and in glassware to enhance durability. Platinum, prized for its exceptional resistance to corrosion and high melting point, has been a go-to material for crucibles, electrodes, and other high-temperature equipment. The contrasting properties of lead and platinum dictated their specific applications, with lead favored for protection and shielding and platinum for precision and chemical resistance.
Safety and Toxicity Considerations
Lead poses significant toxicity risks in laboratory equipment due to its high potential for bioaccumulation and neurotoxicity, necessitating stringent handling and disposal protocols. Platinum, while considerably safer with low toxicity and excellent chemical stability, offers superior resistance to corrosion and contamination in lab settings. Selecting platinum over lead enhances laboratory safety by minimizing hazardous exposure and ensuring reliable, long-lasting performance in sensitive experimental procedures.
Durability and Longevity in Lab Equipment
Platinum exhibits superior durability and longevity compared to lead in laboratory equipment due to its high resistance to corrosion, wear, and chemical reactions. Lead, although inexpensive and easy to shape, tends to degrade faster under acidic or corrosive conditions typical in labs, reducing its lifespan significantly. Platinum's stability under extreme temperatures and harsh environments ensures prolonged equipment functionality and reliability, making it ideal for precision instruments and critical lab applications.
Cost Analysis: Lead vs Platinum
Lead offers significantly lower material costs compared to platinum, making it a more economical choice for laboratory equipment in terms of initial investment. Platinum, while considerably more expensive, provides superior chemical resistance and durability, which can reduce replacement frequency and long-term operational costs. Evaluating total cost of ownership requires balancing lead's affordability against platinum's enhanced performance and longevity in demanding lab environments.
Performance in High-Temperature Applications
Platinum exhibits superior chemical stability and corrosion resistance compared to lead, making it ideal for high-temperature laboratory equipment used in extreme conditions. Its melting point exceeds 1700degC, significantly outperforming lead's 327.5degC, which limits lead's performance in sustained high-heat environments. Enhanced durability and inertness of platinum ensure consistent data accuracy and equipment longevity in critical thermal applications.
Resistance to Chemicals and Corrosion
Platinum demonstrates superior resistance to chemicals and corrosion compared to lead, making it ideal for laboratory equipment exposed to aggressive substances. Lead, while cost-effective, tends to degrade and corrode when in contact with strong acids and bases, limiting its durability in harsh chemical environments. The corrosion-resistant properties of platinum ensure longer equipment lifespan and maintain purity in sensitive lab applications.
Environmental Impact and Sustainability
Lead in laboratory equipment poses significant environmental hazards due to its toxicity and persistence, leading to soil and water contamination during disposal. Platinum, though rare and costly, offers a more sustainable alternative because it is inert, recyclable, and less harmful to ecosystems. Choosing platinum reduces long-term environmental risks and aligns with green laboratory practices focused on sustainability and minimizing hazardous waste.
Choosing the Right Metal: Applications and Recommendations
Platinum offers superior corrosion resistance and high melting points, making it ideal for precise laboratory equipment exposed to harsh chemicals and extreme temperatures, while lead is less durable and primarily used in applications requiring heavy shielding or radiation protection. Choosing the right metal depends on experimental conditions: platinum is preferred for analytical instruments, electrodes, and crucibles where purity and durability are critical, whereas lead suits containment or protective roles without direct chemical exposure. For optimal laboratory performance, prioritize platinum in high-precision and high-stress environments, reserving lead for safety and shielding applications.
Infographic: Lead vs Platinum for Laboratory Equipment
 azmater.com
azmater.com