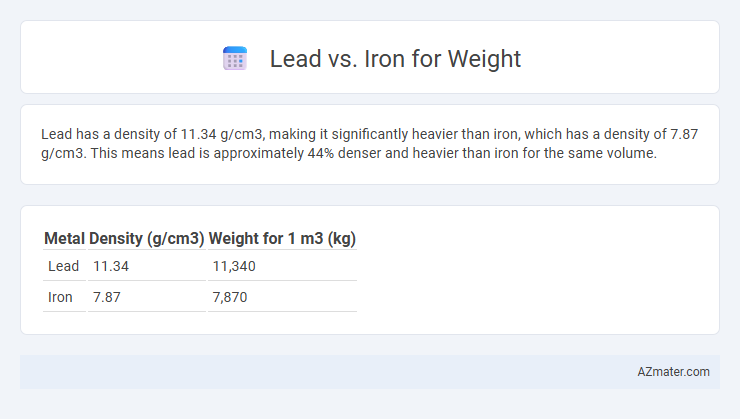
Lead has a density of 11.34 g/cm3, making it significantly heavier than iron, which has a density of 7.87 g/cm3. This means lead is approximately 44% denser and heavier than iron for the same volume.
Table of Comparison
| Metal | Density (g/cm3) | Weight for 1 m3 (kg) |
|---|---|---|
| Lead | 11.34 | 11,340 |
| Iron | 7.87 | 7,870 |
Understanding Lead and Iron: An Overview
Lead has a density of approximately 11.34 grams per cubic centimeter, making it significantly heavier than iron, which has a density of about 7.87 grams per cubic centimeter. This higher density means lead occupies less volume than iron for the same weight, which is essential in applications requiring compactness and high mass, such as radiation shielding. Understanding the differences in weight and density between lead and iron aids in selecting the appropriate material for industrial, construction, and manufacturing purposes.
Physical Properties: Lead vs Iron
Lead has a density of 11.34 g/cm3, making it significantly heavier than iron, which has a density of 7.87 g/cm3. Despite lead's higher density, iron exhibits greater tensile strength and hardness, contributing to its durability in structural applications. Lead's malleability and low melting point contrast with iron's higher melting point of 1538degC, influencing their respective uses in manufacturing and construction.
Weight Comparison: Lead and Iron Density
Lead has a density of approximately 11.34 grams per cubic centimeter, making it significantly heavier than iron, which has a density of around 7.87 grams per cubic centimeter. This means that for the same volume, lead weighs about 44% more than iron. The higher density of lead results from its atomic weight and tightly packed atomic structure, influencing its use in applications requiring high mass in compact forms.
Practical Applications: When to Use Lead or Iron
Lead's high density makes it ideal for applications requiring compact weight, such as radiation shielding, ballast, and soundproofing. Iron, with its lower density but greater strength and magnetic properties, suits structural applications, machinery, and construction where durability and support are crucial. Choosing between lead and iron depends on balancing weight constraints and mechanical performance in specific practical uses.
Cost and Availability of Lead vs Iron
Lead is generally more expensive than iron due to its relative scarcity and higher extraction costs. Iron is abundant and widely available, making it a more cost-effective option for weight applications. The availability of iron in large quantities ensures lower market prices and easier sourcing compared to lead.
Environmental and Health Concerns
Lead poses significant environmental and health risks due to its high toxicity, causing neurotoxicity and contamination in soil and water systems. Iron, while heavier per volume, is less toxic and more environmentally benign, making it a safer choice for applications involving prolonged human exposure. The disposal and recycling of lead require stringent controls to prevent lead poisoning and ecosystem damage, unlike iron, which is more widely recycled with minimal environmental hazards.
Durability and Corrosion Resistance
Lead offers moderate durability but is highly prone to corrosion, especially when exposed to moisture and acidic environments, which limits its long-term use in structural applications. Iron, particularly when alloyed as steel, provides superior durability and enhanced corrosion resistance, especially when treated with protective coatings or alloyed with elements like chromium to form stainless steel. The choice between lead and iron for weight applications depends on the need for structural integrity and resistance to environmental degradation.
Safety Considerations in Handling
Lead, significantly denser than iron with a density of 11.34 g/cm3 compared to iron's 7.87 g/cm3, presents unique safety challenges due to its toxicity and potential for lead poisoning from dust or ingestion. Iron, while heavy and requiring appropriate lifting techniques, does not pose chemical toxicity risks but can cause physical injuries such as cuts or crush injuries during handling. Proper personal protective equipment (PPE), ventilation, and hygiene practices are critical when handling lead to mitigate health risks, whereas ergonomic support is emphasized for safe iron handling to prevent musculoskeletal disorders.
Popular Uses in Industry and Sports
Lead, with its high density of 11.34 g/cm3, is widely used in industries requiring radiation shielding and soundproofing, such as nuclear reactors and construction. Iron, having a lower density of 7.87 g/cm3 but superior tensile strength, dominates in structural applications, automotive manufacturing, and sports equipment like golf clubs and weightlifting bars. The weight advantage of lead makes it ideal for counterweights and ballast, whereas iron's strength-to-weight ratio is preferred for durability in athletic gear.
Choosing the Right Material for Your Needs
Lead is significantly denser than iron, weighing approximately 11.34 grams per cubic centimeter compared to iron's 7.86 grams per cubic centimeter, making lead a better choice for applications requiring compact weight. Iron offers superior strength and durability, ideal for structural uses where weight is less critical but resilience is paramount. Selecting between lead and iron depends on whether you prioritize density for weight or mechanical strength for longevity in your project.
Infographic: Lead vs Iron for Weight
 azmater.com
azmater.com