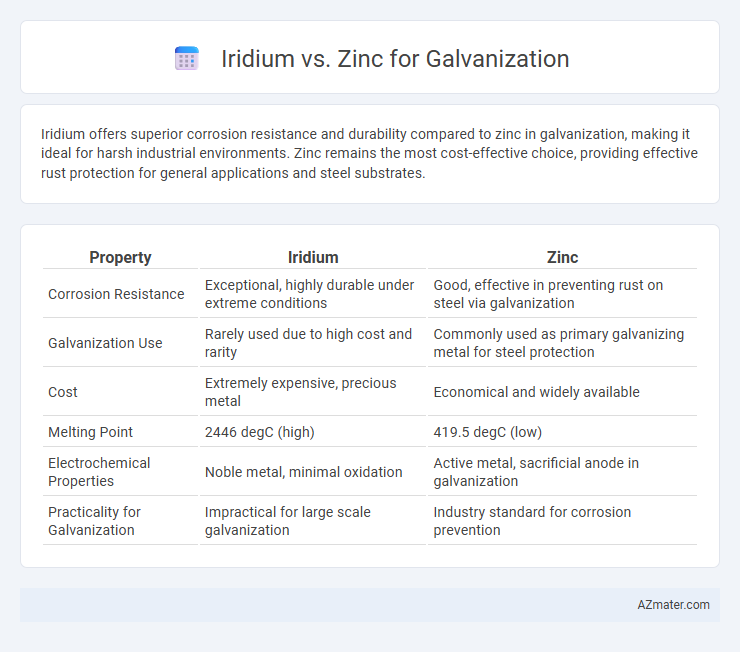
Iridium offers superior corrosion resistance and durability compared to zinc in galvanization, making it ideal for harsh industrial environments. Zinc remains the most cost-effective choice, providing effective rust protection for general applications and steel substrates.
Table of Comparison
| Property | Iridium | Zinc |
|---|---|---|
| Corrosion Resistance | Exceptional, highly durable under extreme conditions | Good, effective in preventing rust on steel via galvanization |
| Galvanization Use | Rarely used due to high cost and rarity | Commonly used as primary galvanizing metal for steel protection |
| Cost | Extremely expensive, precious metal | Economical and widely available |
| Melting Point | 2446 degC (high) | 419.5 degC (low) |
| Electrochemical Properties | Noble metal, minimal oxidation | Active metal, sacrificial anode in galvanization |
| Practicality for Galvanization | Impractical for large scale galvanization | Industry standard for corrosion prevention |
Introduction to Galvanization
Galvanization is a corrosion protection process primarily involving zinc, which forms a durable, sacrificial barrier on steel surfaces. Iridium is rarely used in galvanization due to its high cost and limited electrochemical reactivity compared to zinc. Zinc's electrochemical properties enable it to protect steel effectively by acting as a sacrificial anode, preventing rust and extending the lifespan of metal structures.
Importance of Material Selection in Galvanization
Selecting the appropriate material for galvanization is crucial to ensure optimal corrosion resistance and longevity of the coated metal. While Zinc remains the standard due to its sacrificial anode properties and cost-effectiveness, Iridium offers superior corrosion resistance and durability in extreme environments despite higher costs. The choice between Iridium and Zinc significantly impacts the protective performance, maintenance frequency, and overall lifecycle cost of galvanized steel structures.
Overview of Iridium: Properties and Uses
Iridium is a dense, corrosion-resistant transition metal known for its exceptional hardness and high melting point, making it one of the most durable elements used in industrial applications. Its remarkable resistance to oxidation and chemical attack ensures long-term stability in harsh environments, distinguishing it from other metals like zinc commonly used in galvanization. Iridium's primary uses include high-temperature crucibles, electrical contacts, and catalytic converters, where strength and reliability are critical.
Overview of Zinc: Properties and Uses
Zinc is a lustrous, bluish-white metal known for its excellent corrosion resistance and is widely used in galvanization to protect steel from rusting. Its properties include moderate hardness, good malleability, and a relatively low melting point of 419.5degC, making it ideal for coating applications. Zinc's primary uses extend beyond galvanization to include die casting alloys, battery production, and as a key component in anti-corrosion coatings.
Corrosion Resistance: Iridium vs Zinc
Iridium exhibits exceptional corrosion resistance due to its high chemical inertness and ability to withstand harsh environments, making it superior to zinc in galvanization applications where long-term durability is critical. Zinc, while offering effective sacrificial protection by corroding preferentially and thereby safeguarding the underlying metal, tends to degrade over time under extreme conditions such as acidic or marine atmospheres. The superior stability of iridium coatings makes them ideal for specialized industrial uses requiring minimal maintenance and extended service life compared to traditional zinc galvanization.
Cost Comparison: Iridium vs Zinc for Galvanization
Zinc remains the most cost-effective and widely used metal for galvanization due to its abundant availability and lower market price compared to iridium. Iridium's extremely high price--often thousands of times greater per ounce--renders it economically impractical for large-scale galvanization applications despite its superior corrosion resistance. The significant cost disparity positions zinc as the dominant choice for protective coatings in industrial and construction sectors.
Environmental Impact and Sustainability
Zinc is widely favored for galvanization due to its abundant availability, lower environmental footprint, and effective corrosion resistance, making it a sustainable choice for protecting steel structures. Iridium, though highly corrosion-resistant, is rare and energy-intensive to extract, resulting in a significantly larger environmental impact and limited sustainability for large-scale galvanization. The extensive use of zinc contributes to resource efficiency and reduced ecological disruption compared to the high environmental costs associated with iridium production.
Application Suitability: Industrial and Commercial Uses
Zinc excels in galvanization for industrial and commercial uses due to its cost-effectiveness, excellent corrosion resistance, and widespread availability, making it ideal for steel structures, automotive parts, and construction materials. Iridium, though highly corrosion-resistant, is rarely used for galvanization in industrial applications because of its extreme cost and limited supply, restricting its use primarily to specialized, high-performance coatings in niche sectors. The choice between iridium and zinc for galvanization hinges on project budget, corrosion protection requirements, and application scale.
Longevity and Maintenance Requirements
Iridium offers exceptional longevity in galvanization due to its superior corrosion resistance and hardness, significantly reducing maintenance requirements over time compared to zinc. Zinc, while more affordable and widely used, tends to corrode faster, necessitating more frequent inspections and reapplications to maintain protective effectiveness. The higher durability of iridium coatings ensures prolonged protection in harsh environments, minimizing long-term maintenance costs and downtime.
Conclusion: Best Choice for Galvanization
Zinc remains the best choice for galvanization due to its cost-effectiveness, excellent corrosion resistance, and established industrial use. Iridium, while highly corrosion-resistant, is prohibitively expensive and less practical for large-scale galvanization projects. Zinc coatings provide a reliable sacrificial barrier, extending the lifespan of steel structures efficiently and economically.
Infographic: Iridium vs Zinc for Galvanization
 azmater.com
azmater.com