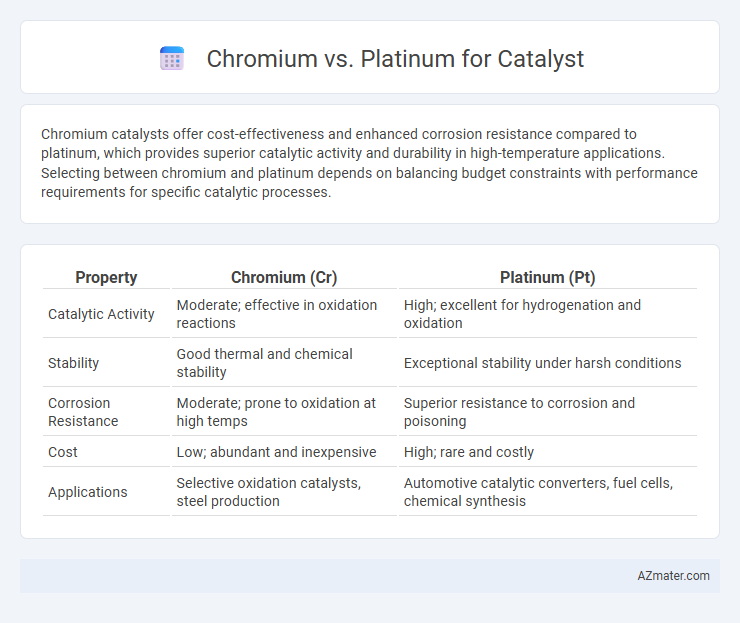
Chromium catalysts offer cost-effectiveness and enhanced corrosion resistance compared to platinum, which provides superior catalytic activity and durability in high-temperature applications. Selecting between chromium and platinum depends on balancing budget constraints with performance requirements for specific catalytic processes.
Table of Comparison
| Property | Chromium (Cr) | Platinum (Pt) |
|---|---|---|
| Catalytic Activity | Moderate; effective in oxidation reactions | High; excellent for hydrogenation and oxidation |
| Stability | Good thermal and chemical stability | Exceptional stability under harsh conditions |
| Corrosion Resistance | Moderate; prone to oxidation at high temps | Superior resistance to corrosion and poisoning |
| Cost | Low; abundant and inexpensive | High; rare and costly |
| Applications | Selective oxidation catalysts, steel production | Automotive catalytic converters, fuel cells, chemical synthesis |
Overview of Catalysts: Chromium vs Platinum
Chromium and platinum serve as essential catalysts in various industrial processes, with chromium commonly used in oxidation and polymerization reactions due to its versatility and cost-effectiveness. Platinum, renowned for its superior catalytic activity and resistance to poisoning, dominates in hydrogenation, reforming, and automotive catalytic converters. The choice between chromium and platinum catalysts hinges on factors like reaction specificity, operating conditions, and economic considerations, balancing performance against material cost and durability.
Chemical Properties and Structure
Chromium exhibits versatile oxidation states, primarily +3 and +6, enabling it to form stable oxides like Cr2O3 that serve as efficient catalysts with high corrosion resistance. Platinum's face-centered cubic (FCC) structure provides excellent catalytic activity due to a large number of surface atoms available for adsorption, making it highly effective in hydrogenation and oxidation reactions. The difference in electronic configuration between chromium (3d5 4s1) and platinum (5d9 6s1) influences their catalytic behavior, with platinum offering superior electron density for facilitating redox reactions at lower temperatures.
Catalytic Efficiency Comparison
Chromium catalysts exhibit high selectivity and thermal stability, making them ideal for ethylene polymerization with moderate activity levels compared to platinum. Platinum catalysts demonstrate superior catalytic efficiency in hydrogenation and oxidation reactions due to their excellent electron transfer properties and resistance to poisoning. The choice between chromium and platinum catalysts hinges on the specific reaction requirements, where chromium offers cost-effective stability and platinum provides enhanced activity and durability.
Cost Analysis: Affordability and Availability
Chromium stands out as a highly cost-effective catalyst material due to its abundant global reserves and lower extraction costs compared to platinum, which is a rare and expensive precious metal. The price volatility of platinum, driven by limited supply and high demand in automotive and jewelry industries, significantly increases its overall cost for catalytic applications. Chromium's affordability and widespread availability make it a preferred choice for large-scale industrial catalysis where economic efficiency is crucial.
Environmental Impact and Sustainability
Chromium catalysts often pose higher environmental risks due to the toxicity and carcinogenic nature of hexavalent chromium compounds, leading to stringent waste management and disposal requirements. Platinum catalysts, despite their higher cost, offer superior sustainability by enabling more efficient reactions with lower energy consumption and generating fewer hazardous byproducts. The recyclability of platinum further enhances its environmental profile, making it a more sustainable choice in catalytic applications compared to chromium.
Industrial Applications and Use Cases
Chromium catalysts excel in industrial applications such as polymerization and chemical synthesis due to their high thermal stability and cost-effectiveness, particularly in ethylene polymer production. Platinum catalysts offer superior catalytic activity and selectivity in processes like automotive catalytic converters and fuel cells, where durability and resistance to poisoning are critical. The choice between chromium and platinum catalysts hinges on specific industrial requirements, balancing factors such as reaction temperature, catalyst lifespan, and economic considerations.
Durability and Longevity in Catalysis
Chromium-based catalysts exhibit high durability due to their resistance to oxidation and thermal degradation, making them suitable for long-term catalytic applications under harsh conditions. Platinum catalysts, while offering superior catalytic activity, often face challenges with agglomeration and poisoning, which can reduce their longevity over time. Advances in alloying platinum with other metals, including chromium, have been employed to enhance both durability and lifespan in catalytic processes.
Poisoning and Deactivation Resistance
Chromium-based catalysts exhibit enhanced resistance to poisoning compared to platinum due to their strong oxygen storage capacity and ability to maintain active sites under harsh conditions. Platinum catalysts, while highly active, are more susceptible to deactivation from sulfur and carbon monoxide poisoning, which blocks active sites and reduces catalytic efficiency. Incorporating chromium improves catalyst durability by minimizing surface contamination and sustaining long-term activity in industrial applications.
Advances in Catalyst Technology
Chromium and platinum catalysts exhibit distinct advances in catalytic performance, with platinum typically offering superior activity and durability in fuel cells and automotive catalytic converters. Recent developments in chromium-based catalysts have enhanced their selectivity and resistance to poisoning, making them a cost-effective alternative for industrial chemical reactions. Nanostructured platinum alloys and chromium oxide composites have driven breakthroughs in catalytic efficiency and environmental sustainability, highlighting a shift towards optimizing metal-support interactions at the atomic scale.
Future Trends and Research Directions
Chromium and platinum catalysts exhibit distinct advantages in catalytic efficiency and durability, with platinum dominating hydrogen fuel cell technologies due to its superior activity and stability. Future research trends emphasize developing chromium-based catalysts as cost-effective alternatives by enhancing their surface chemistry and electronic properties through nanoscale engineering and doping techniques. Advances in computational modeling and in situ characterization aim to optimize catalyst performance while minimizing precious metal usage, driving eco-friendly and economically viable solutions.
Infographic: Chromium vs Platinum for Catalyst
 azmater.com
azmater.com