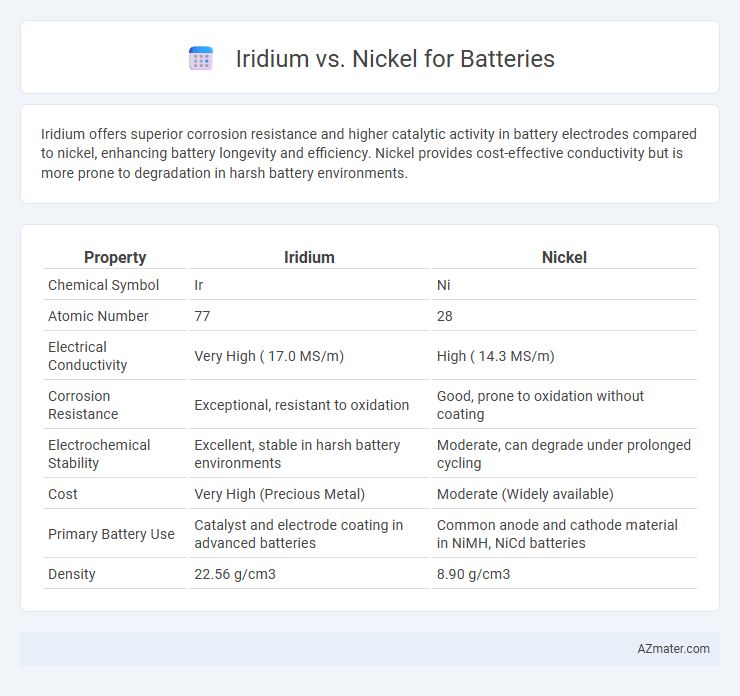
Iridium offers superior corrosion resistance and higher catalytic activity in battery electrodes compared to nickel, enhancing battery longevity and efficiency. Nickel provides cost-effective conductivity but is more prone to degradation in harsh battery environments.
Table of Comparison
| Property | Iridium | Nickel |
|---|---|---|
| Chemical Symbol | Ir | Ni |
| Atomic Number | 77 | 28 |
| Electrical Conductivity | Very High ( 17.0 MS/m) | High ( 14.3 MS/m) |
| Corrosion Resistance | Exceptional, resistant to oxidation | Good, prone to oxidation without coating |
| Electrochemical Stability | Excellent, stable in harsh battery environments | Moderate, can degrade under prolonged cycling |
| Cost | Very High (Precious Metal) | Moderate (Widely available) |
| Primary Battery Use | Catalyst and electrode coating in advanced batteries | Common anode and cathode material in NiMH, NiCd batteries |
| Density | 22.56 g/cm3 | 8.90 g/cm3 |
Overview: Iridium vs Nickel in Battery Technology
Iridium and nickel serve distinct roles in battery technology, with nickel primarily used in nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries due to its high energy density and corrosion resistance. Iridium is less common but crucial in enhancing the durability and catalytic efficiency of certain battery components, such as electrodes in fuel cells and advanced lithium-based batteries. The choice between iridium and nickel depends on performance requirements, cost considerations, and specific battery applications, where nickel offers affordability and energy storage capacity, while iridium provides improved stability and catalytic properties.
Chemical Properties and Reactivity
Iridium exhibits exceptional chemical stability and corrosion resistance due to its inertness and high oxidation states, making it less reactive compared to nickel. Nickel, characterized by variable oxidation states (+2, +3), displays higher chemical reactivity and is prone to oxidation and corrosion in battery environments. These contrasting chemical properties influence their respective roles in battery electrodes, with iridium enhancing durability and nickel contributing to active redox reactions.
Conductivity and Electrochemical Performance
Iridium offers superior electrochemical performance in batteries due to its excellent catalytic activity and corrosion resistance, resulting in higher charge-discharge efficiency and durability. Nickel exhibits high electrical conductivity and is commonly used in battery electrodes, but it is more prone to oxidation and capacity fade over time. The choice between iridium and nickel hinges on balancing iridium's cost-effective performance enhancements against nickel's widespread availability and conductivity benefits.
Cost and Market Availability
Iridium is significantly more expensive than nickel, limiting its widespread use in battery manufacturing despite excellent catalytic properties. Nickel enjoys broad market availability and lower costs, making it a preferred material for large-scale battery production, especially in lithium-ion batteries. The high market demand and cost-effectiveness of nickel drive its dominance over iridium in commercial energy storage solutions.
Environmental Impact and Sustainability
Iridium, a rare and expensive metal primarily used in specialized battery cathodes, has a smaller environmental footprint due to its high efficiency and long cycle life, reducing the frequency of replacements. Nickel, widely employed in lithium-ion batteries, poses significant environmental challenges from mining, including habitat destruction and water pollution, but offers greater material availability and recyclability prospects. Sustainable battery development increasingly emphasizes nickel recycling technologies and iridium's potential in durable, low-impact energy storage solutions to balance resource scarcity with ecological considerations.
Energy Density and Storage Capacity
Iridium offers higher energy density compared to nickel, enabling batteries to store more energy in a smaller volume, which is critical for applications requiring compact and lightweight power sources. Nickel-based batteries, such as nickel-metal hydride (NiMH), provide substantial storage capacity but typically have lower energy density than iridium-enhanced cells. The superior energy density of iridium contributes to longer battery life and improved performance in high-demand electronic devices and electric vehicles.
Durability and Cycle Life
Iridium-based battery electrodes exhibit superior durability and longer cycle life compared to nickel counterparts due to their exceptional corrosion resistance and stable electrochemical properties. Nickel electrodes tend to degrade faster under high charge-discharge rates, limiting battery longevity and performance consistency. Iridium's enhanced stability makes it ideal for applications requiring prolonged cycle life and robust battery durability.
Applications in Modern Batteries
Iridium is primarily used as a catalyst in specialized batteries, enhancing oxygen evolution reactions in fuel cells and certain metal-air batteries due to its excellent corrosion resistance and catalytic activity. Nickel plays a crucial role in nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries, widely applied in portable electronics, power tools, and hybrid vehicles because of its high energy density and stable cycling performance. In modern battery technology, nickel's abundance and cost-effectiveness make it a preferred choice for large-scale applications, whereas iridium's unique electrochemical properties are leveraged in niche, high-performance energy storage systems.
Recent Innovations and Research Trends
Recent innovations in battery technology highlight iridium's enhanced catalytic properties, particularly in oxygen evolution reactions, leading to improved efficiency and durability in rechargeable batteries. Nickel-based batteries continue to dominate due to their cost-effectiveness and energy density, but research is increasingly exploring nickel-iron and nickel-cobalt alloys to optimize performance and cycle life. Current trends emphasize hybrid cathode materials combining iridium and nickel to leverage iridium's stability and nickel's conductivity for advanced battery systems.
Future Prospects and Industry Outlook
Iridium and nickel play distinct roles in battery technology, with nickel dominating as a key component in lithium-ion cathodes due to its energy density advantages and cost-efficiency. Iridium's future prospects lie primarily in durable, high-performance applications like solid-state and advanced flow batteries, where its excellent catalytic properties enhance longevity and efficiency. Industry outlook suggests nickel will continue to lead in mass-market EV batteries, while iridium-based technologies gain traction in specialized, high-end energy storage solutions.
Infographic: Iridium vs Nickel for Battery
 azmater.com
azmater.com