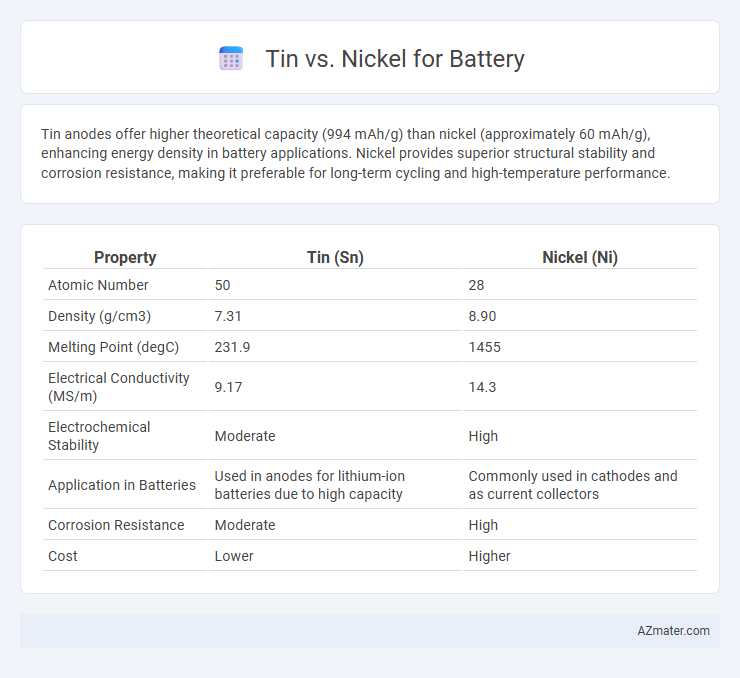
Tin anodes offer higher theoretical capacity (994 mAh/g) than nickel (approximately 60 mAh/g), enhancing energy density in battery applications. Nickel provides superior structural stability and corrosion resistance, making it preferable for long-term cycling and high-temperature performance.
Table of Comparison
| Property | Tin (Sn) | Nickel (Ni) |
|---|---|---|
| Atomic Number | 50 | 28 |
| Density (g/cm3) | 7.31 | 8.90 |
| Melting Point (degC) | 231.9 | 1455 |
| Electrical Conductivity (MS/m) | 9.17 | 14.3 |
| Electrochemical Stability | Moderate | High |
| Application in Batteries | Used in anodes for lithium-ion batteries due to high capacity | Commonly used in cathodes and as current collectors |
| Corrosion Resistance | Moderate | High |
| Cost | Lower | Higher |
Introduction to Tin and Nickel in Battery Technology
Tin and nickel are critical materials in battery technology, extensively used to enhance electrode performance and energy density. Tin, prized for its high theoretical capacity and low cost, is commonly employed in anode materials to improve lithium-ion storage capabilities, though it undergoes significant volume expansion during charge cycles. Nickel, favored for its high conductivity and stability, is widely utilized in cathode compositions such as nickel-cobalt-manganese (NCM) and nickel-cobalt-aluminum (NCA) oxides, contributing to increased battery lifespan and energy output.
Chemical Properties: Tin vs Nickel in Batteries
Tin exhibits excellent electrochemical properties such as high theoretical capacity (~994 mAh/g) and good alloying behavior with lithium, making it a promising anode material in lithium-ion batteries. Nickel, while having a lower capacity (~290 mAh/g), offers superior chemical stability and conductivity, enabling efficient electron transfer and longer cycle life in battery cathodes. The choice between tin and nickel depends on balancing high energy density with chemical stability and cycle performance in battery applications.
Energy Density Comparison
Tin-based anodes in batteries offer higher theoretical energy densities compared to nickel due to tin's ability to alloy with more lithium ions, enabling greater charge storage capacity. Nickel-based cathodes, although stable and widely used, typically provide lower energy density per unit mass than tin anodes, which can lead to improved battery performance in terms of capacity and runtime. The enhanced volumetric and gravimetric energy density of tin anodes contributes to the development of high-capacity lithium-ion batteries aimed at longer-lasting energy storage solutions.
Charge/Discharge Efficiency
Tin anodes demonstrate higher charge/discharge efficiency compared to nickel due to their superior lithium-ion storage capacity and lower volumetric expansion during cycling. Nickel's lower efficiency results from its limited lithium absorption and greater susceptibility to structural degradation, causing capacity fade. Optimizing tin-based materials significantly enhances battery performance by reducing energy loss and improving cycle stability.
Cycle Life and Durability
Tin anodes offer higher theoretical capacity compared to nickel, but they suffer from significant volume expansion during charge-discharge cycles, leading to reduced cycle life and mechanical degradation. Nickel-based batteries exhibit superior durability with stable electrochemical performance over extended cycling, maintaining structural integrity under repeated stress. Advanced electrode designs incorporating tin-nickel composites aim to balance capacity and cycle stability, enhancing overall battery lifespan.
Cost Analysis: Tin vs Nickel Batteries
Tin-based batteries generally offer a lower production cost compared to nickel-based batteries due to the abundance and cheaper extraction process of tin. Nickel batteries, while more expensive upfront, provide higher energy density and longer life cycles, which can reduce long-term operational costs. Evaluating total cost of ownership, including raw material prices, manufacturing, and performance sustainability, is crucial to determine the most cost-effective choice between tin and nickel battery technologies.
Environmental Impact and Recycling
Tin used in batteries offers significant environmental advantages over nickel due to its abundance and lower toxicity, reducing ecological risks during extraction and disposal. Recycling tin from batteries is more energy-efficient and generates fewer harmful byproducts compared to nickel recycling, which often involves complex processes and toxic waste management. Transitioning to tin-based battery anodes enhances sustainability by promoting circular economy principles and minimizing environmental degradation associated with nickel mining and processing.
Safety Considerations
Tin-based anodes offer improved safety over nickel due to lower risk of thermal runaway and reduced dendrite formation during charging cycles. Nickel's higher reactivity can lead to increased heat generation and potential short-circuits, posing greater hazards in battery applications. Safety considerations favor tin in terms of stability, reduced flammability, and enhanced cycle life in lithium-ion batteries.
Industry Applications and Trends
Tin and nickel play critical roles in battery technologies, with tin increasingly used in anodes for lithium-ion batteries due to its high theoretical capacity and ability to improve energy density, making it attractive for electric vehicles and portable electronics. Nickel remains essential in cathodes, especially in nickel-rich chemistries such as NMC (Nickel Manganese Cobalt) and NCA (Nickel Cobalt Aluminum), driving higher energy output and cycle life in automotive and grid storage applications. Current trends emphasize the development of tin-based composite anodes to mitigate volume expansion and nickel cathodes with reduced cobalt content to enhance sustainability and reduce costs in large-scale battery production.
Future Prospects: Innovations with Tin and Nickel
Emerging innovations in battery technology highlight tin's high theoretical capacity and abundant supply as key advantages for future anode materials in lithium-ion batteries, promising increased energy density and faster charging times. Nickel continues to dominate cathode chemistry, particularly in nickel-rich NMC (nickel-manganese-cobalt) materials, improving energy density and cycle life for electric vehicle batteries. Advances such as silicon-tin composites for anodes and stabilized nickel-rich cathodes suggest ongoing enhancements that could revolutionize performance, cost, and sustainability in next-generation energy storage solutions.
Infographic: Tin vs Nickel for Battery
 azmater.com
azmater.com