Iridium offers superior corrosion resistance and high melting point compared to aluminum, making it ideal for high-performance alloying applications. Aluminum provides lightweight properties and excellent electrical conductivity but lacks the durability and strength of iridium in extreme environments.
Table of Comparison
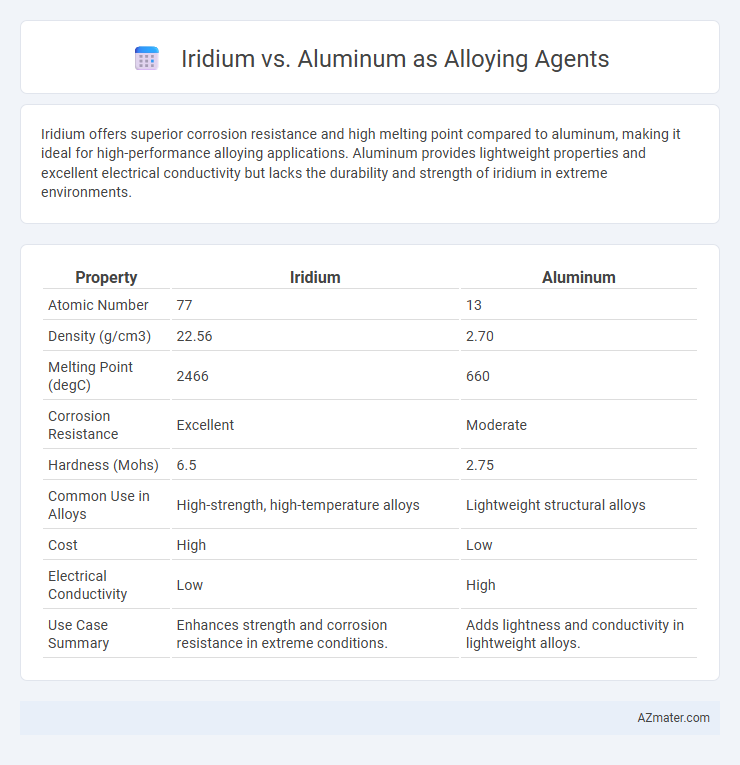
| Property | Iridium | Aluminum |
|---|---|---|
| Atomic Number | 77 | 13 |
| Density (g/cm3) | 22.56 | 2.70 |
| Melting Point (degC) | 2466 | 660 |
| Corrosion Resistance | Excellent | Moderate |
| Hardness (Mohs) | 6.5 | 2.75 |
| Common Use in Alloys | High-strength, high-temperature alloys | Lightweight structural alloys |
| Cost | High | Low |
| Electrical Conductivity | Low | High |
| Use Case Summary | Enhances strength and corrosion resistance in extreme conditions. | Adds lightness and conductivity in lightweight alloys. |
Introduction to Alloying Agents: Iridium vs Aluminum
Iridium and aluminum serve distinct roles as alloying agents, with iridium primarily enhancing hardness, corrosion resistance, and high-temperature stability in platinum and other noble metal alloys. Aluminum acts as a lightweight alloying element that improves strength, corrosion resistance, and castability in alloys such as aluminum-silicon and aluminum-copper systems. The choice between iridium and aluminum depends on application requirements, where iridium is favored for extreme environments while aluminum is selected for lightweight structural applications.
Chemical Properties of Iridium and Aluminum
Iridium exhibits exceptional chemical stability, resisting corrosion and oxidation due to its high density and strong atomic bonds, making it ideal for alloying in high-temperature and harsh environments. Aluminum offers excellent chemical reactivity with oxygen, forming a protective oxide layer that enhances corrosion resistance and contributes to its lightweight nature in alloys. The combination of iridium's inert chemical properties with aluminum's oxidation resistance creates alloys with superior durability and strength in challenging chemical conditions.
Physical Characteristics: Strength, Weight, and Durability
Iridium exhibits exceptional strength and durability, outperforming aluminum by maintaining structural integrity under extreme conditions, making it ideal for high-stress alloy applications. Despite being significantly denser and heavier than aluminum, iridium's superior corrosion resistance enhances longevity in harsh environments. Aluminum alloys offer lightweight properties and moderate strength, suitable for applications where weight reduction is critical but sacrifice some durability compared to iridium-based alloys.
Melting Points and Thermal Stability
Iridium exhibits a significantly higher melting point of approximately 2,446degC compared to aluminum's melting point of 660.3degC, making iridium more suitable for high-temperature alloy applications. Its exceptional thermal stability allows iridium-containing alloys to maintain mechanical strength and resist oxidation in extreme heat environments, unlike aluminum alloys which tend to soften and degrade. Therefore, iridium is preferred in aerospace and industrial sectors where thermal resistance and durability at elevated temperatures are critical.
Corrosion Resistance Comparison
Iridium offers superior corrosion resistance compared to aluminum when used as alloying agents, especially in extreme environments due to its excellent resistance to oxidation and chemical attack. Aluminum alloys tend to form a protective oxide layer that prevents further corrosion but are more susceptible to pitting and galvanic corrosion in aggressive media. The inherent stability and inertness of iridium make it a preferred choice for applications demanding long-term durability and minimal degradation under harsh chemical exposure.
Industrial Applications: Iridium vs Aluminum Alloys
Iridium alloys exhibit exceptional corrosion resistance, high melting points, and superior tensile strength, making them ideal for aerospace components and catalytic converters in harsh industrial environments. Aluminum alloys, prized for their lightweight, excellent thermal conductivity, and cost-effectiveness, dominate automotive and construction industries where weight reduction and affordability are crucial. The choice between iridium and aluminum alloys hinges on balancing performance requirements with material costs and application-specific durability demands.
Cost and Economic Considerations
Iridium, known for its extreme rarity and high melting point, commands significantly higher costs compared to aluminum, making it economically impractical for large-scale alloying despite its superior corrosion resistance and strength. Aluminum, abundant and cost-effective, offers excellent lightweight properties and corrosion resistance, making it the preferred choice for alloying in industries focused on cost-efficiency and mass production. The substantial price difference, with iridium costing thousands of dollars per ounce versus aluminum's fraction of a dollar per pound, heavily influences alloy selection based on budget constraints and application scale.
Environmental Impact and Sustainability
Iridium, a rare and highly corrosion-resistant metal, offers superior durability and longevity in alloy applications, reducing the need for frequent replacements and lowering environmental waste. Aluminum, abundant and highly recyclable, boasts a lower energy footprint for production and recycling, making it a more sustainable choice for large-scale alloying. While iridium's extraction has significant environmental impacts due to mining rarity, aluminum's widespread availability and established recycling infrastructure contribute to its overall sustainability in alloy manufacturing.
Availability and Resource Extraction
Iridium, a rare platinum-group metal, is significantly less abundant than aluminum, which ranks as the third most abundant element in the Earth's crust. The extraction of iridium is complex, often recovered as a byproduct of nickel and platinum mining, resulting in limited supply and higher costs. Aluminum is extracted primarily through bauxite ore refining, a well-established, large-scale industrial process that ensures broad availability for alloy production.
Future Trends in Alloying: Iridium or Aluminum?
Iridium and aluminum present distinct advantages as alloying agents, with iridium offering exceptional corrosion resistance and high-temperature stability ideal for aerospace and electronics, while aluminum provides lightweight properties and cost-effectiveness favored in automotive and construction industries. Future trends indicate growing demand for iridium in specialized, high-performance alloys, driven by advancements in catalytic converters and medical devices, whereas aluminum continues to dominate in sustainable and lightweight material development due to its recyclability and abundance. Innovations in nanotechnology and additive manufacturing are expected to enhance the performance characteristics of both, but the choice will heavily depend on application-specific requirements and economic factors.
Infographic: Iridium vs Aluminum for Alloying Agent
 azmater.com
azmater.com