Antimony enhances battery performance with higher energy density and improved cycle stability compared to lead, which offers lower cost but reduced durability. Advanced antimony-based batteries exhibit greater charge retention and faster response times, making them ideal for high-demand applications.
Table of Comparison
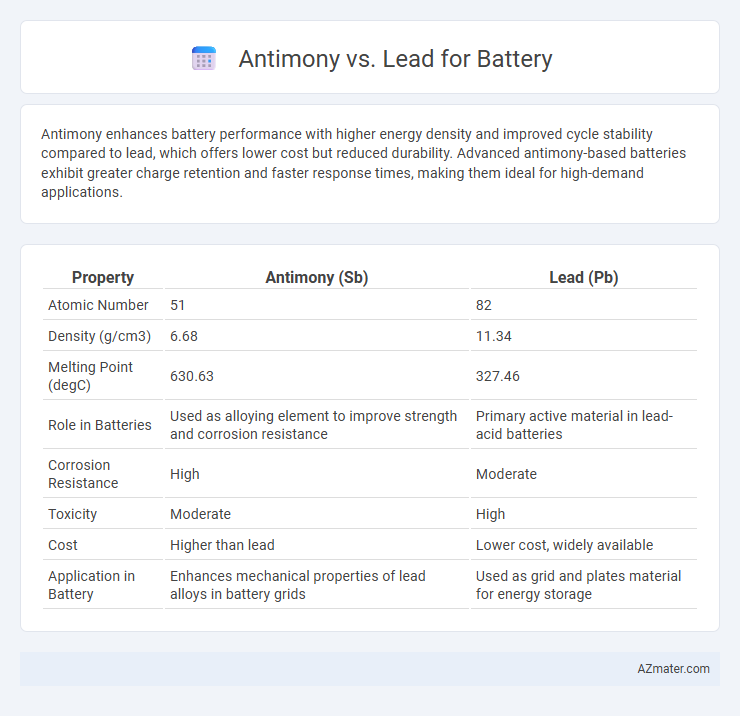
| Property | Antimony (Sb) | Lead (Pb) |
|---|---|---|
| Atomic Number | 51 | 82 |
| Density (g/cm3) | 6.68 | 11.34 |
| Melting Point (degC) | 630.63 | 327.46 |
| Role in Batteries | Used as alloying element to improve strength and corrosion resistance | Primary active material in lead-acid batteries |
| Corrosion Resistance | High | Moderate |
| Toxicity | Moderate | High |
| Cost | Higher than lead | Lower cost, widely available |
| Application in Battery | Enhances mechanical properties of lead alloys in battery grids | Used as grid and plates material for energy storage |
Introduction to Antimony and Lead in Batteries
Antimony and lead are key components in the manufacturing of lead-acid batteries, where lead serves as the primary active material in the plates, and antimony is commonly alloyed with lead to enhance the battery's durability and performance. Lead provides excellent electrochemical properties for energy storage, while antimony improves mechanical strength, corrosion resistance, and grid rigidity in the battery plates. The combination of antimony and lead optimizes battery lifespan and charge efficiency, making them essential materials in automotive and industrial battery applications.
Chemical Properties: Antimony vs Lead
Antimony exhibits higher hardness and corrosion resistance compared to lead, making it valuable for enhancing the durability of battery grids. Lead's lower melting point and superior electrical conductivity facilitate efficient energy storage and discharge in lead-acid batteries. The differing chemical stability and alloying behavior of antimony and lead directly impact the battery's performance, lifespan, and maintenance requirements.
Battery Performance and Efficiency Differences
Antimony-enhanced lead-acid batteries exhibit improved charge acceptance and cycle life due to antimony's ability to refine the grid structure and reduce corrosion. Lead batteries without antimony tend to have lower mechanical strength and shorter lifespan but offer slightly higher purity, which can affect energy efficiency. The inclusion of antimony typically results in higher self-discharge rates, affecting overall efficiency but enhancing durability and performance in deep-cycle applications.
Cost Comparison: Antimony vs Lead
Antimony batteries typically incur higher initial material costs compared to lead batteries due to the price of antimony metal, which is more expensive and subject to market fluctuations. Lead batteries benefit from a well-established supply chain and lower raw material costs, making them more economical for large-scale applications. However, the longer lifespan and enhanced performance of antimony alloy batteries can offset higher upfront expenses by reducing maintenance and replacement costs over time.
Environmental Impact and Toxicity
Antimony in batteries poses moderate environmental risks due to its toxicity and persistence in ecosystems, with potential bioaccumulation affecting aquatic life. Lead, widely used in traditional batteries, presents higher toxicity concerns, causing severe environmental contamination and health hazards through soil and water pollution. Recycling practices for lead batteries are well-established but require strict regulation to minimize lead exposure and environmental damage.
Durability and Lifespan Considerations
Antimony-infused lead alloys enhance battery durability by improving grid strength and corrosion resistance, resulting in longer lifespan compared to pure lead batteries. Batteries with antimony exhibit superior cyclic stability, reducing the frequency of replacements in deep-cycle applications. Lead-acid batteries with low or no antimony content tend to have shorter service life due to increased grid corrosion and grid growth under high-temperature conditions.
Applications in Battery Technologies
Antimony enhances the mechanical strength and cycle life of lead-acid batteries by forming alloys with lead, improving grid durability and corrosion resistance, which is vital for automotive and industrial applications. Lead remains the primary active material in battery electrodes due to its high electrical conductivity and electrochemical stability, essential for energy storage in backup power systems. The integration of antimony with lead optimizes battery performance by balancing weight, cost, and lifespan, making it a preferred choice in advanced valve-regulated lead-acid (VRLA) batteries.
Recyclability and Sustainability Factors
Antimony in batteries offers higher recyclability due to its ability to be efficiently recovered and reused with minimal environmental impact compared to lead. Lead recycling is well-established but poses sustainability challenges given its toxic nature and energy-intensive processes. Utilizing antimony enhances battery sustainability by reducing hazardous waste and supporting circular economy practices in energy storage solutions.
Market Trends and Future Prospects
Antimony is gaining traction in battery technology due to its ability to enhance lead-acid battery performance by increasing energy density and cycle life, appealing to the expanding renewable energy storage market. Lead remains dominant in traditional battery applications, especially automotive and industrial sectors, supported by established recycling infrastructure and cost-effectiveness. Market trends indicate growing investment in antimony-lead alloy development to improve battery efficiency, with future prospects centered on meeting the increasing demand for sustainable and high-performance energy storage solutions.
Choosing the Right Material for Batteries
Antimony offers enhanced corrosion resistance and improved mechanical strength, making it ideal for lead-acid battery grids that require durability and long life cycles. Lead remains a popular choice due to its high electrical conductivity and cost-effectiveness, but its softness may compromise structural integrity over time. Selecting between antimony and lead hinges on balancing performance needs with budget constraints, where antimony's superior alloy properties cater to high-performance applications while pure lead suits cost-sensitive use cases.
Infographic: Antimony vs Lead for Battery
 azmater.com
azmater.com