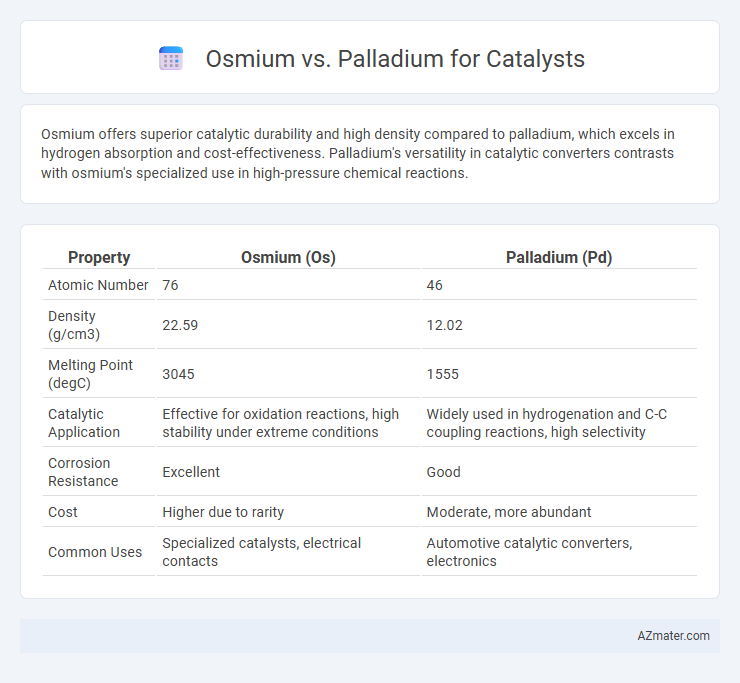
Osmium offers superior catalytic durability and high density compared to palladium, which excels in hydrogen absorption and cost-effectiveness. Palladium's versatility in catalytic converters contrasts with osmium's specialized use in high-pressure chemical reactions.
Table of Comparison
| Property | Osmium (Os) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 76 | 46 |
| Density (g/cm3) | 22.59 | 12.02 |
| Melting Point (degC) | 3045 | 1555 |
| Catalytic Application | Effective for oxidation reactions, high stability under extreme conditions | Widely used in hydrogenation and C-C coupling reactions, high selectivity |
| Corrosion Resistance | Excellent | Good |
| Cost | Higher due to rarity | Moderate, more abundant |
| Common Uses | Specialized catalysts, electrical contacts | Automotive catalytic converters, electronics |
Introduction to Osmium and Palladium as Catalysts
Osmium and palladium are transition metals widely studied for their catalytic properties in chemical reactions. Osmium, known for its high density and oxidation resistance, exhibits strong catalytic activity in hydrogenation and oxidation processes. Palladium is highly valued for its efficient catalytic performance in carbon-carbon coupling reactions and hydrogenation, making it a key component in automotive catalytic converters and fine chemical synthesis.
Chemical Properties Relevant to Catalysis
Osmium exhibits a high density of active surface sites and exceptional catalytic stability due to its strong metal-metal bonding and resistance to oxidation, making it ideal for redox reactions and hydrogenation processes. Palladium features superior affinity for hydrogen adsorption and facile electron transfer, which enhances its performance in catalytic hydrogenation and carbon-carbon coupling reactions. Both metals demonstrate unique electronic configurations and surface chemistries that significantly influence reaction activation energies and turnover frequencies in heterogeneous catalysis.
Abundance and Sourcing Comparison
Osmium and palladium serve as effective catalysts, but their abundance and sourcing differ significantly, impacting availability and cost. Palladium is more abundant in the Earth's crust, with an estimated concentration of about 0.015 parts per million, primarily sourced from South Africa, Russia, and Canada through established mining operations. Osmium is rarer, found at approximately 0.001 parts per million, mostly obtained as a byproduct of platinum and nickel mining, which limits its supply and increases market scarcity.
Catalytic Efficiency in Key Reactions
Osmium exhibits superior catalytic efficiency in oxidation reactions due to its high density and robust electron transfer capabilities, enabling faster conversion rates and greater selectivity. Palladium excels in hydrogenation and carbon-carbon coupling reactions, with its versatile electronic structure facilitating strong substrate binding and activation. Comparative studies reveal that while osmium catalysts often outperform palladium in oxidative environments, palladium remains preferred for processes requiring mild reaction conditions and recyclability.
Selectivity and Reaction Specificity
Osmium exhibits higher selectivity in hydrogenation reactions due to its unique electronic structure, enabling precise control over reaction pathways and minimizing by-products. Palladium, while highly active, often shows broader catalytic activity, which can lead to lower reaction specificity but greater versatility across different substrates. The choice between osmium and palladium catalysts depends on the desired balance between selectivity and reaction scope for fine chemical synthesis.
Stability and Resistance to Deactivation
Osmium exhibits superior stability and resistance to deactivation in catalytic applications due to its high melting point (3045degC) and robust atomic structure, which minimize catalyst sintering and poisoning. Palladium, while effective as a catalyst, tends to deactivate more rapidly under harsh conditions because of its lower melting point (1554degC) and susceptibility to sulfur and carbonaceous deposits. These differences make osmium catalysts preferable for long-term, high-temperature reactions requiring sustained activity and durability.
Industrial Applications and Use Cases
Osmium exhibits exceptional catalytic properties in industrial oxidation reactions, particularly in the manufacture of specialty chemicals and fine organic synthesis due to its high density and chemical stability. Palladium is widely favored in catalytic converters for automotive emission control and hydrogenation processes in pharmaceutical manufacturing, owing to its excellent selectivity and resistance to poisoning. Both metals serve crucial roles in industrial catalysis but palladium's broader adoption is driven by cost efficiency and availability compared to the rarer, more expensive osmium.
Environmental and Safety Considerations
Osmium and palladium are both used as catalysts with distinct environmental and safety profiles. Osmium, particularly as osmium tetroxide, poses significant toxicity risks and requires careful handling due to its volatility and potential to cause respiratory and skin damage. Palladium catalysts are generally safer and more environmentally friendly, with lower toxicity and better recyclability, making them preferred in green chemistry applications.
Cost and Economic Feasibility
Osmium, a rare and dense transition metal, generally exhibits higher catalytic activity but is significantly more expensive and less abundant than palladium, impacting its economic feasibility for large-scale industrial use. Palladium, widely used in catalytic converters and chemical reactions, offers a balanced cost-to-performance ratio with relatively lower prices and greater availability, making it a more practical choice for most commercial catalyst applications. Cost-efficiency and supply stability position palladium as the preferred material, while osmium's high cost and scarcity limit its usage despite superior catalytic properties.
Future Trends and Innovations in Catalyst Research
Osmium exhibits exceptional catalytic properties due to its high density and stability, making it ideal for specific high-precision reactions, while palladium remains dominant in catalytic converters and hydrogenation processes due to its excellent activity and lower cost. Innovations in catalyst research focus on enhancing durability and selectivity, with emerging trends exploring osmium-palladium alloys and nanostructured catalysts to improve efficiency and reduce precious metal usage. Future developments also aim to leverage osmium's resistance to corrosion and palladium's versatility to create hybrid catalysts for sustainable chemical manufacturing and fuel cell technologies.
Infographic: Osmium vs Palladium for Catalyst
 azmater.com
azmater.com