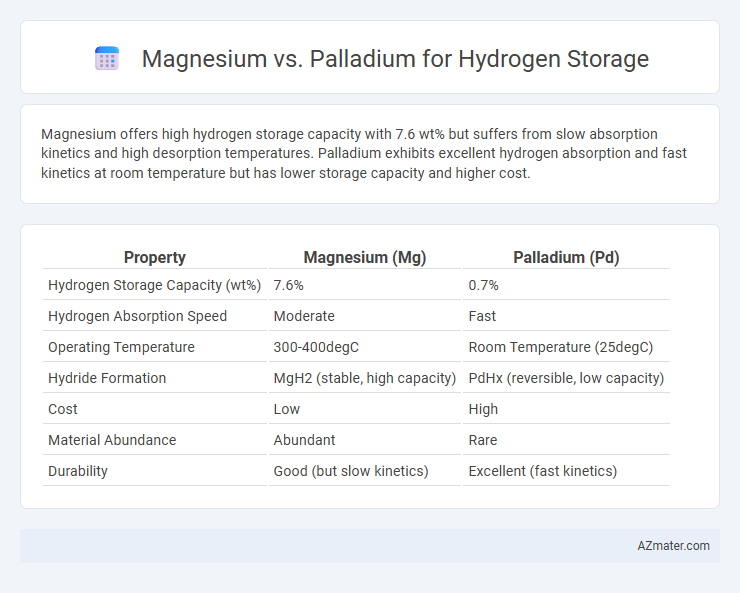
Magnesium offers high hydrogen storage capacity with 7.6 wt% but suffers from slow absorption kinetics and high desorption temperatures. Palladium exhibits excellent hydrogen absorption and fast kinetics at room temperature but has lower storage capacity and higher cost.
Table of Comparison
| Property | Magnesium (Mg) | Palladium (Pd) |
|---|---|---|
| Hydrogen Storage Capacity (wt%) | 7.6% | 0.7% |
| Hydrogen Absorption Speed | Moderate | Fast |
| Operating Temperature | 300-400degC | Room Temperature (25degC) |
| Hydride Formation | MgH2 (stable, high capacity) | PdHx (reversible, low capacity) |
| Cost | Low | High |
| Material Abundance | Abundant | Rare |
| Durability | Good (but slow kinetics) | Excellent (fast kinetics) |
Introduction to Hydrogen Storage Materials
Magnesium and palladium are prominent materials in hydrogen storage due to their distinct absorption properties and storage capacities. Magnesium offers high hydrogen storage capacity through hydride formation, making it suitable for large-scale applications despite slower kinetics. Palladium facilitates rapid hydrogen absorption and release at lower pressures, favored in sensor and purification technologies.
Overview of Magnesium and Palladium Properties
Magnesium exhibits high hydrogen storage capacity due to its ability to form stable metal hydrides, with a capacity of up to 7.6 wt%, making it cost-effective and abundant for large-scale applications. Palladium offers exceptional hydrogen absorption properties, including rapid kinetics and high selectivity, but at a premium cost and limited storage capacity of around 1.4 wt%. The differing thermodynamic and kinetic behaviors of magnesium and palladium dictate their suitability for hydrogen storage, with magnesium favoring high-capacity bulk storage and palladium excelling in sensor and purification technologies.
Hydrogen Absorption Mechanisms
Magnesium stores hydrogen through the formation of metal hydrides by absorbing hydrogen atoms into its lattice structure, enabling a high storage capacity but requiring elevated temperatures for absorption and desorption. Palladium exhibits a rapid hydrogen absorption mechanism through its ability to dissociate hydrogen molecules into atoms on the surface, allowing hydrogen to diffuse interstitially into its face-centered cubic lattice at ambient conditions. The differences in thermodynamics and kinetics make magnesium suitable for high-capacity storage with slower uptake, while palladium offers quicker hydrogen absorption with lower storage capacity.
Storage Capacity Comparison
Magnesium offers a high hydrogen storage capacity of up to 7.6 wt%, making it a promising material for efficient hydrogen storage, while palladium typically stores hydrogen at a lower capacity of around 0.6 wt%. The difference in storage capacity primarily stems from magnesium's ability to form stable hydrides, whereas palladium absorbs hydrogen through surface adsorption and lattice expansion. Despite its lower capacity, palladium exhibits faster hydrogen absorption and desorption kinetics, which is beneficial for applications requiring rapid hydrogen uptake and release.
Kinetics of Hydrogen Uptake and Release
Magnesium exhibits high hydrogen storage capacity but suffers from slow kinetics in hydrogen uptake and release, often requiring elevated temperatures above 300degC to enhance reaction rates. Palladium demonstrates superior kinetics at near-ambient temperatures due to its excellent catalytic properties, enabling rapid hydrogen absorption and desorption, albeit with significantly lower storage capacity compared to magnesium. Research increasingly focuses on alloying magnesium or using nanostructured composites to improve its hydrogen sorption kinetics while leveraging palladium's fast hydrogen diffusion pathways.
Operating Conditions and Safety Considerations
Magnesium offers high hydrogen storage capacity at elevated temperatures around 300-400degC and moderate pressures, but its slow absorption kinetics and pyrophoric risk demand careful thermal management and protective atmospheres. Palladium operates efficiently at near-ambient temperatures and lower pressures with rapid hydrogen uptake and release, providing safer handling but limited storage capacity due to high material cost and weight. Safety considerations favor palladium for low-risk, reversible hydrogen storage, while magnesium requires stringent controls to prevent oxidation and embrittlement under harsh operating conditions.
Material Cost and Availability Analysis
Magnesium offers a significantly lower material cost compared to palladium, making it a more economically viable option for large-scale hydrogen storage applications. Its abundant availability in the Earth's crust ensures a steady supply, whereas palladium is scarce and subject to price volatility due to limited natural reserves and high demand in various industries. The cost-effectiveness and abundant supply of magnesium make it preferable over palladium for cost-sensitive hydrogen storage solutions.
Durability and Cycling Performance
Magnesium offers high hydrogen storage capacity but faces challenges in durability due to particle pulverization and slow kinetics during cycling, leading to capacity degradation over time. Palladium exhibits superior cycling performance and excellent durability, maintaining structural integrity through numerous hydrogen absorption-desorption cycles because of its ability to resist embrittlement and phase changes. The trade-off between magnesium's high storage density and palladium's long-term stability defines their suitability for practical hydrogen storage applications.
Recent Advances and Research Trends
Recent advances in hydrogen storage highlight magnesium's high gravimetric capacity and cost-effectiveness, with nanostructuring and alloying techniques improving its absorption kinetics and cycle stability. Palladium excels in hydrogen sorption due to its exceptional catalytic properties and rapid absorption-desorption cycles, though its high cost and limited storage capacity restrict widespread application. Research trends focus on hybrid materials combining magnesium's storage capacity with palladium's catalytic efficiency to achieve enhanced hydrogen storage performance under moderate conditions.
Future Prospects for Hydrogen Storage Technologies
Magnesium's high hydrogen storage capacity of around 7.6 wt% positions it as a promising material for large-scale and cost-effective hydrogen storage solutions, though its slow absorption kinetics and high operating temperatures remain challenges. Palladium offers exceptional hydrogen absorption at low temperatures and rapid kinetics but suffers from high cost and limited storage capacity, restricting its use to niche applications like sensing and purification. Future hydrogen storage technologies are exploring composite materials and nanostructured alloys combining magnesium's capacity with palladium's kinetics to achieve efficient, durable, and economically viable hydrogen storage systems.
Infographic: Magnesium vs Palladium for Hydrogen Storage
 azmater.com
azmater.com