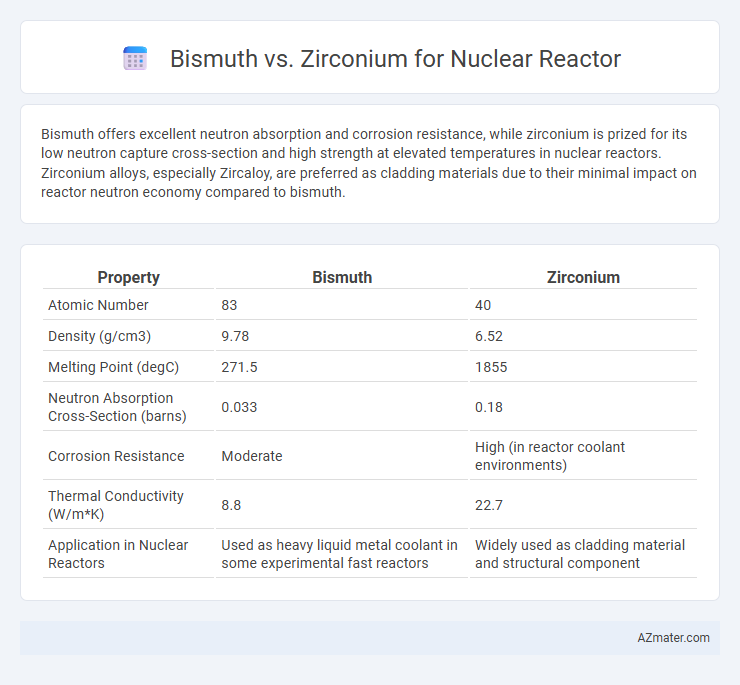
Bismuth offers excellent neutron absorption and corrosion resistance, while zirconium is prized for its low neutron capture cross-section and high strength at elevated temperatures in nuclear reactors. Zirconium alloys, especially Zircaloy, are preferred as cladding materials due to their minimal impact on reactor neutron economy compared to bismuth.
Table of Comparison
| Property | Bismuth | Zirconium |
|---|---|---|
| Atomic Number | 83 | 40 |
| Density (g/cm3) | 9.78 | 6.52 |
| Melting Point (degC) | 271.5 | 1855 |
| Neutron Absorption Cross-Section (barns) | 0.033 | 0.18 |
| Corrosion Resistance | Moderate | High (in reactor coolant environments) |
| Thermal Conductivity (W/m*K) | 8.8 | 22.7 |
| Application in Nuclear Reactors | Used as heavy liquid metal coolant in some experimental fast reactors | Widely used as cladding material and structural component |
Introduction to Bismuth and Zirconium in Nuclear Reactors
Bismuth and zirconium serve distinct roles in nuclear reactors, with zirconium primarily used as cladding material for fuel rods due to its low neutron absorption and excellent corrosion resistance. Bismuth, with its high atomic number and density, is employed mainly in spallation targets and as a coolant in certain advanced reactor designs because of its good thermal conductivity and low neutron absorption. The choice between bismuth and zirconium depends on the reactor type and specific operational requirements, balancing factors such as neutron economy, thermal properties, and corrosion behavior.
Material Properties: Bismuth vs Zirconium
Bismuth and zirconium exhibit distinct material properties crucial for nuclear reactor applications, with zirconium offering superior corrosion resistance and a low neutron absorption cross-section, essential for fuel cladding in light water reactors. Bismuth possesses a high density and excellent thermal conductivity but suffers from brittleness and chemical instability under reactor conditions. Zirconium's strength at high temperatures and its ability to form a protective oxide layer make it the preferred material for maintaining structural integrity in nuclear environments.
Nuclear Reactivity and Neutron Absorption Comparison
Bismuth exhibits low neutron absorption cross-section, making it advantageous for minimizing neutron capture and maintaining nuclear reactivity in reactor coolant systems, whereas zirconium's extremely low thermal neutron absorption (~0.18 barns) significantly contributes to efficient neutron economy in fuel cladding applications. Zirconium alloys are preferred for fuel cladding due to their structural integrity and minimal impact on neutron flux, while bismuth's higher atomic number leads to greater neutron inelastic scattering. The choice between bismuth and zirconium hinges on balancing neutron absorption properties with mechanical performance requirements in specific reactor environments.
Corrosion Resistance under Reactor Conditions
Bismuth exhibits excellent corrosion resistance under high-temperature reactor conditions due to its low neutron absorption cross-section and chemical stability, making it suitable for certain fast reactor coolants. Zirconium, widely used in nuclear reactors as cladding material, offers superior corrosion resistance by forming a stable and protective oxide layer (ZrO2) that withstands prolonged exposure to high-temperature water and radiation. While zirconium's corrosion resistance is well-documented in light water reactors, bismuth's application is more niche, often favored in liquid metal fast reactors for its corrosive inertness and compatibility with fuel elements.
Thermal Conductivity and Heat Transfer Efficiency
Bismuth exhibits lower thermal conductivity compared to zirconium, impacting its heat transfer efficiency in nuclear reactors. Zirconium alloys are preferred for fuel cladding due to their higher thermal conductivity, enhancing heat dissipation from the reactor core. Efficient heat transfer in zirconium-based materials contributes to improved reactor safety and performance under high-temperature conditions.
Mechanical Strength and Durability Analysis
Bismuth exhibits relatively low mechanical strength and limited durability under high neutron flux, making it less suitable for structural components in nuclear reactors compared to zirconium. Zirconium alloys demonstrate excellent mechanical strength, corrosion resistance, and radiation tolerance, which ensures long-term durability in reactor environments. Consequently, zirconium is preferred for fuel cladding and structural applications due to its superior ability to withstand mechanical stress and maintain integrity during prolonged reactor operation.
Radioactive Behavior and Waste Management
Bismuth exhibits lower neutron absorption rates compared to zirconium, resulting in reduced radioactivation during reactor operation, which simplifies radioactive waste management. Zirconium alloys, widely used in cladding materials, generate more long-lived radioactive isotopes like Zr-93, complicating waste disposal and requiring advanced containment strategies. Bismuth's favorable radioactive behavior and lower activation make it a promising candidate in reducing nuclear waste radioactivity levels and enhancing overall reactor safety.
Economic and Supply Chain Considerations
Bismuth offers a cost-effective alternative to zirconium in nuclear reactors due to its lower market price and abundant availability, which helps mitigate supply chain disruptions. Zirconium, while more expensive, benefits from established global supply chains and processing infrastructure that ensure consistent quality and reliability. Economic assessments reveal that incorporating bismuth can reduce raw material expenses but may introduce challenges in sourcing high-purity grades required for reactor applications.
Applications in Modern and Advanced Reactor Designs
Bismuth, with its high atomic number and excellent neutron absorption properties, is predominantly used in fast breeder reactors as a coolant and neutron reflector, enhancing neutron economy and heat transfer efficiency. Zirconium alloys are essential in modern nuclear reactors for cladding fuel rods due to their low neutron absorption cross-section and superior corrosion resistance under high-temperature reactor conditions. Advanced reactor designs increasingly rely on zirconium's compatibility with various coolants, while bismuth's role is specialized in liquid metal fast reactors and some lead-bismuth eutectic systems for improved thermal conductivity and neutron moderation.
Future Prospects and Research Directions
Bismuth's excellent neutron absorption properties and low melting point make it a promising candidate for advanced nuclear reactor cooling systems, particularly in fast reactors and lead-bismuth eutectic coolant designs, while zirconium remains critical for fuel cladding due to its low neutron absorption and high corrosion resistance. Future research is directed at enhancing bismuth-based coolants' thermal conductivity and corrosion resistance to improve reactor safety and efficiency, alongside developing zirconium alloys with improved hydrogen embrittlement resistance and radiation tolerance. Innovations in additive manufacturing and surface treatments for zirconium alloys, combined with experimental and modeling studies on bismuth's thermophysical behavior under irradiation, aim to optimize material performance in next-generation nuclear reactors.
Infographic: Bismuth vs Zirconium for Nuclear reactor
 azmater.com
azmater.com