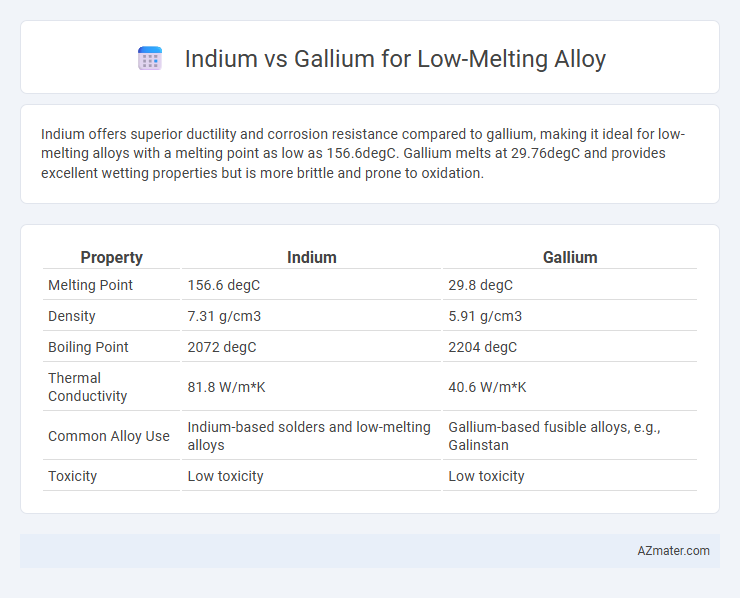
Indium offers superior ductility and corrosion resistance compared to gallium, making it ideal for low-melting alloys with a melting point as low as 156.6degC. Gallium melts at 29.76degC and provides excellent wetting properties but is more brittle and prone to oxidation.
Table of Comparison
| Property | Indium | Gallium |
|---|---|---|
| Melting Point | 156.6 degC | 29.8 degC |
| Density | 7.31 g/cm3 | 5.91 g/cm3 |
| Boiling Point | 2072 degC | 2204 degC |
| Thermal Conductivity | 81.8 W/m*K | 40.6 W/m*K |
| Common Alloy Use | Indium-based solders and low-melting alloys | Gallium-based fusible alloys, e.g., Galinstan |
| Toxicity | Low toxicity | Low toxicity |
Introduction to Low-Melting Alloys
Indium and gallium are key elements in low-melting alloys due to their unique melting points--indium melts at 156.6degC while gallium melts at just 29.8degC, making them valuable for applications requiring precise thermal control. Indium-based alloys often provide superior wettability and ductility, whereas gallium alloys excel in non-toxicity and fluidity at near-room temperatures. Both metals enable the development of fusible alloys for electronics, thermal interface materials, and safety devices, enhancing performance across temperature-sensitive industries.
Overview of Indium and Gallium
Indium and gallium are critical metals commonly used in low-melting alloys due to their unique physical properties. Indium features a melting point of 156.6degC and exhibits excellent wetting capabilities, making it ideal for bonding and thermal interface materials. Gallium melts at a lower temperature of 29.76degC and offers superior fluidity and low toxicity, enhancing its use in thermometers, semiconductors, and fusible alloy applications.
Melting Points: Indium vs Gallium
Indium has a melting point of 156.6degC, making it suitable for low-melting alloys that require stability above room temperature, while Gallium melts at just 29.76degC, allowing for alloys that melt near or slightly above room temperature. The significant difference in melting points allows Gallium-based alloys to remain liquid at much lower temperatures compared to Indium-based alloys, which generally offer higher thermal resistance. Selecting between Indium and Gallium depends on the desired melting temperature range and the specific thermal properties required for applications such as thermal interface materials or fusible alloys.
Alloy Formation Capabilities
Indium and gallium both exhibit excellent alloy formation capabilities, but indium offers superior wetting properties with a wider range of metals, making it ideal for low-melting alloys in electronics and soldering applications. Gallium's low melting point and ability to form eutectic alloys with aluminum and other metals provide unique advantages for thermal management and flexible electronics. The choice between indium and gallium depends on specific alloy performance requirements, including melting temperature, mechanical strength, and chemical compatibility.
Thermal and Electrical Conductivity
Indium exhibits superior thermal conductivity around 81.8 W/m*K and electrical conductivity near 1.2 x 10^7 S/m, making it highly efficient for low-melting alloys requiring rapid heat dissipation and reliable electrical performance. Gallium has a lower thermal conductivity at approximately 29 W/m*K and electrical conductivity about 7.1 x 10^6 S/m, which limits its effectiveness in applications demanding high conductivity. Choosing indium over gallium enhances alloy performance in electronic cooling and conductive pathways due to its better ability to manage thermal and electrical loads.
Mechanical Properties and Ductility
Indium exhibits superior ductility and excellent mechanical flexibility compared to gallium, making it more suitable for low-melting alloys requiring high deformation tolerance. Gallium, while having a lower melting point, tends to be more brittle and less mechanically resilient in alloy form. The choice between indium and gallium for low-melting alloys hinges on the need for enhanced ductility and reliable mechanical performance in applications such as thermal interface materials and flexible electronics.
Corrosion Resistance and Chemical Stability
Indium exhibits superior corrosion resistance and chemical stability compared to gallium in low-melting alloys, making it more suitable for applications requiring long-term durability in harsh environments. Gallium tends to oxidize and react more readily with moisture and oxygen, which can degrade alloy performance over time. The enhanced stability of indium alloys contributes to their reliability in electronic soldering and thermal interface materials.
Applications in Electronics and Industry
Indium and gallium are critical metals for low-melting alloys used in electronics and industrial applications, with indium providing excellent thermal and electrical conductivity in solders and thermal interface materials. Gallium's low melting point and non-toxicity make it ideal for liquid metal cooling systems and flexible electronics, where precise thermal management and electrical performance are essential. Both metals enable advancements in semiconductor manufacturing, with indium-tin alloys used in transparent conductive coatings and gallium-based alloys supporting next-generation optoelectronic devices.
Cost and Availability Considerations
Indium is significantly more expensive and less abundant compared to gallium, making gallium a more cost-effective choice for low-melting alloys. Gallium's wider availability and lower price contribute to its increased adoption in applications such as thermal interfaces and fusible alloys. The economic advantage of gallium helps offset performance differences in low-melting-temperature materials used in electronics and specialized metallurgy.
Environmental and Safety Factors
Indium and gallium both serve as key components in low-melting alloys, but indium boasts lower toxicity and reduced environmental impact compared to gallium, which can pose moderate health risks upon prolonged exposure. Indium's lower evaporation rate at working temperatures minimizes inhalation hazards, enhancing workplace safety in alloy processing and applications. Proper handling and disposal protocols remain crucial for both metals to prevent environmental contamination and ensure operator safety.
Infographic: Indium vs Gallium for Low-melting Alloy
 azmater.com
azmater.com