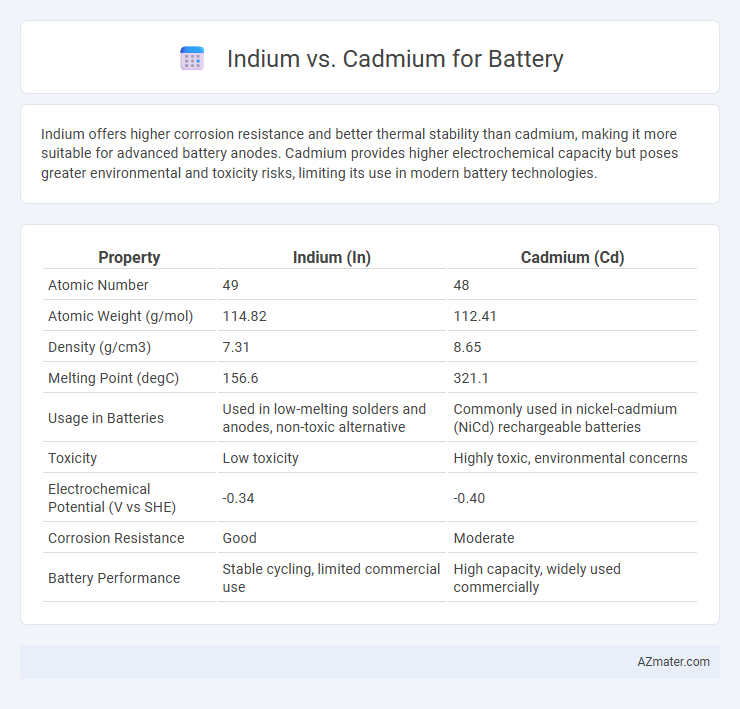
Indium offers higher corrosion resistance and better thermal stability than cadmium, making it more suitable for advanced battery anodes. Cadmium provides higher electrochemical capacity but poses greater environmental and toxicity risks, limiting its use in modern battery technologies.
Table of Comparison
| Property | Indium (In) | Cadmium (Cd) |
|---|---|---|
| Atomic Number | 49 | 48 |
| Atomic Weight (g/mol) | 114.82 | 112.41 |
| Density (g/cm3) | 7.31 | 8.65 |
| Melting Point (degC) | 156.6 | 321.1 |
| Usage in Batteries | Used in low-melting solders and anodes, non-toxic alternative | Commonly used in nickel-cadmium (NiCd) rechargeable batteries |
| Toxicity | Low toxicity | Highly toxic, environmental concerns |
| Electrochemical Potential (V vs SHE) | -0.34 | -0.40 |
| Corrosion Resistance | Good | Moderate |
| Battery Performance | Stable cycling, limited commercial use | High capacity, widely used commercially |
Introduction to Indium and Cadmium in Battery Technology
Indium and cadmium are critical elements used in battery technology due to their unique electrochemical properties. Indium enhances conductivity and stability in advanced battery electrodes, while cadmium is primarily utilized in nickel-cadmium (NiCd) batteries, known for their high cycle life and reliability. The distinct roles of indium and cadmium influence battery performance, safety, and environmental impact in energy storage applications.
Chemical Properties and Elemental Overview
Indium exhibits a melting point of 156.6degC and belongs to group 13 with a stable +3 oxidation state, making it suitable for improving conductivity in battery electrodes. Cadmium, with a melting point of 321degC and group 12 classification, primarily functions in rechargeable nickel-cadmium (NiCd) batteries due to its stable +2 oxidation state and high electrochemical potential. The differing chemical reactivities and elemental properties influence their application, where indium enhances battery stability and cadmium offers reliable energy density.
Role of Indium in Modern Batteries
Indium plays a crucial role in modern batteries by enhancing the electrochemical stability and conductivity of battery electrodes, particularly in lithium-ion and thin-film solar batteries. Its ability to form stable alloys and compounds improves the lifespan and charge efficiency of batteries compared to cadmium, which is more toxic and less conductive. Indium's integration in battery technology supports safer, longer-lasting energy storage solutions with reduced environmental impact.
Role of Cadmium in Traditional Batteries
Cadmium plays a critical role in traditional nickel-cadmium (Ni-Cd) batteries by serving as the active material in the negative electrode, offering high discharge rates and durability. Despite its toxicity and environmental concerns, cadmium's electrochemical properties enable stable charge retention and reliable cycle life in rechargeable battery applications. Innovations addressing cadmium's environmental impact have led to decreased usage, but it remains essential in some legacy battery technologies for portable power solutions.
Energy Density Comparison: Indium vs Cadmium
Indium-based batteries typically exhibit higher energy density than cadmium counterparts due to indium's superior electrochemical properties and lighter atomic weight, enabling more efficient energy storage per unit mass. Cadmium, commonly used in nickel-cadmium (NiCd) batteries, offers robust cycle life but generally lower energy density, which limits its application in high-capacity energy storage systems. Advanced indium-based electrode designs leverage the metal's higher capacity to improve battery performance in portable electronics and renewable energy storage solutions.
Environmental Impact and Toxicity Concerns
Indium and cadmium differ significantly in environmental impact and toxicity when used in batteries. Cadmium is highly toxic and poses substantial environmental hazards due to its persistence and bioaccumulation, leading to severe health risks such as kidney damage and carcinogenic effects. Indium, while less toxic, still requires careful handling and recycling to mitigate environmental contamination, but it is generally considered a safer alternative in battery technologies.
Cost and Availability of Indium and Cadmium
Indium is significantly rarer and more expensive than cadmium, with indium prices averaging around $400 per kilogram compared to cadmium's price of approximately $10 per kilogram. Cadmium, primarily sourced as a byproduct of zinc mining, benefits from more abundant availability and established recycling streams, making it a cost-effective option for battery production. Indium's limited global supply and concentration in a few countries contribute to its higher cost and potential supply chain constraints in battery manufacturing.
Performance and Lifespan Differences
Indium batteries typically exhibit higher energy density and better thermal stability compared to cadmium-based batteries, which results in enhanced performance in high-drain applications. Cadmium batteries, such as nickel-cadmium (NiCd), offer robust cycle life but suffer from memory effect, leading to reduced effective capacity over time. Indium's corrosion resistance extends battery lifespan by minimizing electrode degradation, making it more suitable for long-term energy storage solutions.
Current Applications and Market Trends
Indium is primarily used in thin-film battery technologies due to its excellent conductivity and corrosion resistance, enhancing battery efficiency and longevity in flexible electronics and wearable devices. Cadmium is mainly employed in nickel-cadmium (NiCd) rechargeable batteries, which are favored for their robustness and stable performance in power tools and emergency lighting despite environmental concerns. Market trends indicate a shift away from cadmium batteries due to toxicity regulations, while indium's role is expanding in emerging battery segments driven by demand for lightweight, high-capacity energy storage solutions.
Future Prospects: Indium vs Cadmium in Battery Innovation
Indium offers promising future prospects in battery innovation due to its high conductivity and stability, making it ideal for advanced lithium-ion and solid-state batteries. Cadmium, despite its historical use in nickel-cadmium (NiCd) batteries, faces decline because of toxicity concerns and regulatory restrictions limiting widespread adoption. Emerging research prioritizes indium-based materials for safer, longer-lasting, and environmentally friendly battery technologies, positioning indium as a critical element in next-generation energy storage solutions.
Infographic: Indium vs Cadmium for Battery
 azmater.com
azmater.com