Hafnium-based catalysts exhibit superior thermal stability and resistance to sintering compared to palladium, enhancing catalytic efficiency in high-temperature reactions. Palladium catalysts offer exceptional hydrogen absorption and selectivity, making them ideal for hydrogenation processes.
Table of Comparison
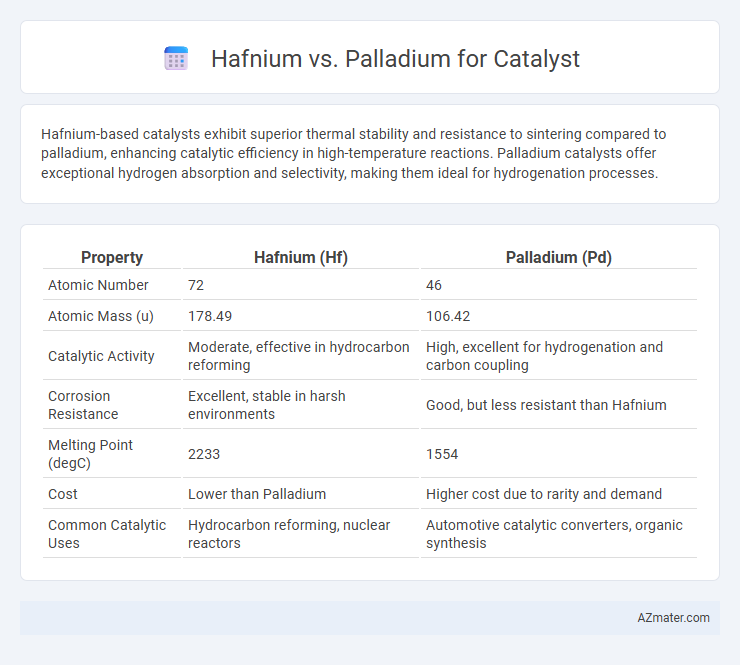
| Property | Hafnium (Hf) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 72 | 46 |
| Atomic Mass (u) | 178.49 | 106.42 |
| Catalytic Activity | Moderate, effective in hydrocarbon reforming | High, excellent for hydrogenation and carbon coupling |
| Corrosion Resistance | Excellent, stable in harsh environments | Good, but less resistant than Hafnium |
| Melting Point (degC) | 2233 | 1554 |
| Cost | Lower than Palladium | Higher cost due to rarity and demand |
| Common Catalytic Uses | Hydrocarbon reforming, nuclear reactors | Automotive catalytic converters, organic synthesis |
Introduction to Hafnium and Palladium as Catalysts
Hafnium and palladium serve distinct roles as catalysts in chemical reactions, with hafnium known for its high thermal stability and strong Lewis acidity, making it effective in polymerization and hydrocarbon transformations. Palladium is widely recognized for its exceptional catalytic activity in cross-coupling reactions, hydrogenation, and oxidation processes, often utilized in pharmaceuticals and fine chemicals production. Comparing their catalytic properties highlights palladium's versatility in organic synthesis versus hafnium's specialized performance in high-temperature and organometallic catalysis.
Chemical Properties Relevant to Catalysis
Hafnium exhibits strong oxidation resistance and a high melting point, which contribute to its stability as a catalyst under extreme reaction conditions, while its ability to form various coordination complexes enhances catalytic activity in hydrocarbon reforming. Palladium, known for its exceptional hydrogen absorption and affinity, is highly effective in catalytic hydrogenation and carbon-carbon coupling reactions due to its unfilled d-orbitals facilitating electron transfer. The differing electronic configurations and chemical reactivities of hafnium ([Xe]4f14 5d2 6s2) and palladium ([Kr]4d10) define their unique catalytic behaviors, with palladium favoring surface reactions and hafnium excelling in high-temperature catalytic environments.
Availability and Cost Comparison
Hafnium and palladium serve as catalysts in various chemical reactions, but their availability significantly impacts their cost and industrial use. Hafnium is more abundant in the Earth's crust, primarily extracted as a byproduct of zirconium refining, making it relatively less expensive than palladium, which is a rare and precious metal mined mainly in South Africa and Russia. Palladium's scarcity and high demand in automotive catalytic converters drive its price higher, while hafnium's lower cost makes it an attractive alternative in applications where cost efficiency is critical.
Catalytic Efficiency in Industrial Processes
Hafnium exhibits superior thermal stability and resistance to corrosion compared to palladium, enhancing catalytic efficiency in high-temperature industrial processes such as hydrocarbon reforming and ammonia synthesis. Palladium's notable hydrogen absorption capacity and selectivity make it highly effective in catalytic converters and hydrogenation reactions, but its performance diminishes at elevated temperatures where hafnium excels. Industrial applications requiring prolonged catalyst lifespan and robustness often favor hafnium-based catalysts for optimized reaction rates and reduced deactivation.
Thermal Stability and Durability
Hafnium-based catalysts exhibit superior thermal stability compared to palladium, maintaining structural integrity at temperatures exceeding 900degC, which makes them ideal for high-temperature industrial applications. Palladium catalysts, while effective at lower temperatures, tend to sinter and lose activity when exposed to prolonged thermal stress above 600degC, reducing their durability. The enhanced thermal endurance of hafnium catalysts contributes to longer operational lifespans and lower regeneration frequency in catalytic converters and chemical reactors.
Selectivity and Reaction Specificity
Hafnium catalysts exhibit superior selectivity and reaction specificity in hydrogenation processes due to their unique electronic structure and robust metal-support interactions, enabling precise control over activation sites. Palladium catalysts, while highly active, often display broader reactivity profiles that can lead to less selective product formation in multifunctional substrates. The controlled use of hafnium enhances targeted transformations, reducing side reactions and improving overall catalytic efficiency in fine chemical synthesis.
Environmental Impact and Sustainability
Hafnium-based catalysts exhibit a lower environmental footprint due to their high durability and resistance to corrosion, reducing the frequency of replacement and waste generation compared to palladium. Palladium, while effective in catalytic converters, faces sustainability challenges from limited natural reserves and energy-intensive mining processes that contribute to habitat destruction and greenhouse gas emissions. Employing hafnium can enhance catalyst lifecycle sustainability by minimizing resource depletion and lowering the overall ecological impact of industrial catalytic reactions.
Common Applications in Catalysis
Hafnium and palladium are widely used catalysts but serve distinct roles due to their unique properties. Hafnium is valued in hydrocarbon reforming and polymerization processes, enhancing reaction rates through strong Lewis acid sites, while palladium excels in hydrogenation, carbon-carbon coupling, and automotive catalytic converters by facilitating efficient electron transfer and adsorption of hydrogen. Their catalytic efficiency is leveraged in the chemical, pharmaceutical, and automotive industries, with palladium dominating in cross-coupling reactions like Suzuki and Heck, whereas hafnium is preferred for olefin polymerization and isomerization.
Challenges and Limitations of Each Metal
Hafnium catalysts face challenges related to their scarcity and high cost, limiting large-scale industrial applications despite excellent thermal stability and resistance to corrosion. Palladium catalysts encounter limitations from susceptibility to poisoning by sulfur and carbonaceous deposits, which reduce catalytic efficiency and lifespan. Both metals also require precise control of particle size and dispersion to optimize activity, impacting manufacturing complexity and cost.
Future Prospects in Catalyst Development
Hafnium exhibits promising potential in catalyst development due to its high thermal stability, corrosion resistance, and ability to facilitate selective hydrogenation reactions, making it suitable for harsh reaction environments. Palladium remains a cornerstone in catalytic applications, particularly in hydrogenation and carbon-carbon coupling reactions, but its high cost and susceptibility to poisoning drive research toward alternatives like hafnium. Future prospects emphasize integrating hafnium-based catalysts with nanostructured materials to enhance active site availability and catalytic efficiency, positioning hafnium as a competitive candidate for sustainable and cost-effective catalysis.
Infographic: Hafnium vs Palladium for Catalyst
 azmater.com
azmater.com