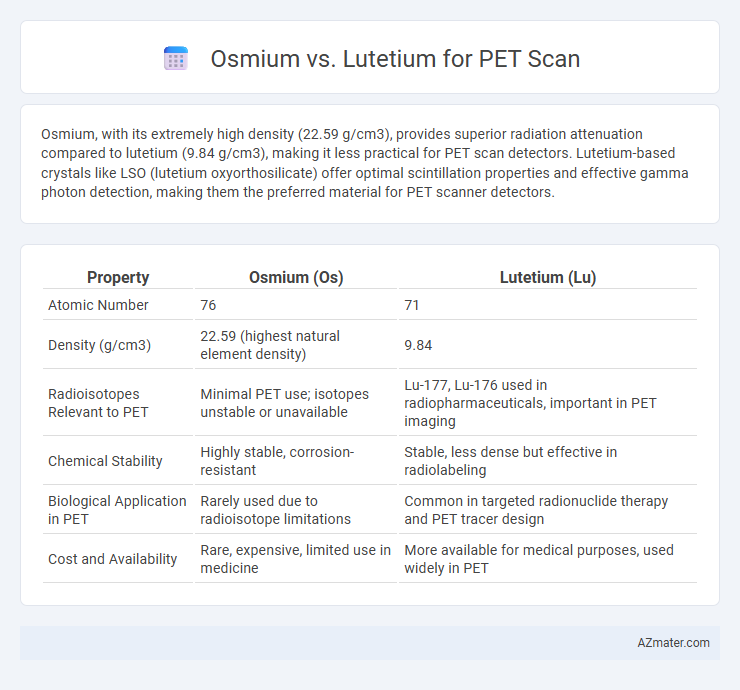
Osmium, with its extremely high density (22.59 g/cm3), provides superior radiation attenuation compared to lutetium (9.84 g/cm3), making it less practical for PET scan detectors. Lutetium-based crystals like LSO (lutetium oxyorthosilicate) offer optimal scintillation properties and effective gamma photon detection, making them the preferred material for PET scanner detectors.
Table of Comparison
| Property | Osmium (Os) | Lutetium (Lu) |
|---|---|---|
| Atomic Number | 76 | 71 |
| Density (g/cm3) | 22.59 (highest natural element density) | 9.84 |
| Radioisotopes Relevant to PET | Minimal PET use; isotopes unstable or unavailable | Lu-177, Lu-176 used in radiopharmaceuticals, important in PET imaging |
| Chemical Stability | Highly stable, corrosion-resistant | Stable, less dense but effective in radiolabeling |
| Biological Application in PET | Rarely used due to radioisotope limitations | Common in targeted radionuclide therapy and PET tracer design |
| Cost and Availability | Rare, expensive, limited use in medicine | More available for medical purposes, used widely in PET |
Introduction to PET Scans: Role of Radiotracers
PET scans utilize radiotracers to detect metabolic activity and diagnose diseases at the molecular level, with elements like Osmium and Lutetium being explored for their unique radioactive properties. Lutetium-177, a commonly used radiotracer, offers optimal decay characteristics and tissue penetration for imaging and targeted therapy. Osmium isotopes, though less conventional, present potential advantages in specific tracer development due to their distinct electron configurations and half-lives.
Overview of Osmium and Lutetium in Medical Imaging
Osmium and lutetium are critical elements utilized in medical imaging with specific roles in positron emission tomography (PET) scanning. Osmium, known for its high density and stability, contributes to the development of contrast agents that enhance image resolution in PET scans. Lutetium, especially in the form of lutetium-177, is prominent in targeted radiotherapy and PET imaging due to its favorable radioactive decay properties and ability to bind with biological molecules for precise tumor detection.
Chemical Properties Relevant to PET Applications
Osmium and Lutetium exhibit distinct chemical properties that influence their suitability for PET scan applications, with Lutetium being more commonly utilized due to its favorable radioactive isotopes such as Lutetium-177, which emits beta and gamma radiation ideal for imaging and therapy. Osmium, characterized by its high density and stability, lacks suitable radioactive isotopes with optimal decay properties for PET imaging, limiting its use in medical diagnostics. Lutetium's chemical reactivity allows for stable complex formation with chelators in radiopharmaceuticals, enhancing targeted delivery and image resolution in PET scans.
Radioisotope Availability and Production
Osmium radioisotopes are rarely used in PET scans due to scarce availability and complex production processes, limiting their practical application. Lutetium-177 is more commonly employed, benefiting from well-established production methods in cyclotrons and nuclear reactors, ensuring consistent supply for radiopharmaceutical synthesis. The widespread accessibility of Lutetium-177's radioisotopes makes them preferable for targeted radiotherapy and PET imaging compared to Osmium counterparts.
Imaging Efficacy: Osmium vs Lutetium
Osmium and lutetium differ significantly in imaging efficacy for PET scans due to their distinct physical and chemical properties. Lutetium-based isotopes, such as Lu-177, are favored for their optimal positron emission and half-life, providing high-resolution images with efficient decay profiles suitable for clinical diagnostics. Osmium isotopes have limited use in PET imaging due to lower positron yield and less favorable decay characteristics, resulting in reduced image clarity and diagnostic accuracy compared to lutetium.
Safety Profiles and Toxicity Concerns
Osmium and Lutetium exhibit distinct safety profiles and toxicity concerns when used in PET scans, with Lutetium being favored due to its lower toxicity and established biocompatibility in radiopharmaceutical applications. Osmium, particularly in its osmium tetroxide form, poses significant toxicity risks, including respiratory and cellular damage, limiting its clinical use compared to Lutetium-177, which is widely used for targeted radiotherapy with manageable side effects. Lutetium's radiological properties combined with a well-understood safety profile make it a preferred choice for diagnostic and therapeutic PET procedures.
Cost and Accessibility of Osmium and Lutetium
Lutetium-based isotopes, such as Lutetium-177, are more commonly used in PET scans due to their established availability and lower cost compared to Osmium isotopes, which are rare and expensive to produce. Osmium's limited accessibility and high extraction costs restrict its practical application in PET imaging. Lutetium's widespread use in targeted radiotherapy and its relatively affordable production make it the preferred choice for cost-effective and accessible PET diagnostics.
Regulatory Considerations in Radiopharmaceutical Use
Osmium and Lutetium compounds in PET scans present distinct regulatory challenges due to their differing radiochemical properties and stability profiles. Lutetium-177, widely used in targeted radiotherapy, benefits from established regulatory frameworks ensuring its safety, efficacy, and quality, whereas osmium isotopes lack comprehensive regulatory guidance due to their rarity and limited clinical use. Regulatory agencies emphasize stringent compliance with radiopharmaceutical manufacturing standards, dosimetry, and patient safety protocols, making lutetium the preferred choice under current radiopharmaceutical regulations for PET scan applications.
Future Trends in PET Scan Radiotracer Development
Osmium and Lutetium are emerging elements in PET scan radiotracer research, with Lutetium-177 already established in theranostic applications due to its favorable half-life and beta emissions. Future trends emphasize the development of Lutetium-based radiotracers for targeted alpha therapy combined with PET imaging, enhancing precision in cancer diagnosis and treatment monitoring. Osmium's potential lies in its unique oxidation states enabling novel chelation chemistry, which could improve radiotracer stability and specificity in the next generation of PET probes.
Conclusion: Selecting the Optimal Element for PET Imaging
Osmium and lutetium both offer unique properties for PET scan applications, with lutetium providing superior positron emission characteristics and biocompatibility crucial for effective imaging. Osmium's higher atomic number contributes to better attenuation correction but poses challenges in radiolabeling and toxicity. Selecting lutetium aligns with enhanced image resolution and patient safety, making it the optimal element for PET imaging protocols.
Infographic: Osmium vs Lutetium for PET Scan
 azmater.com
azmater.com