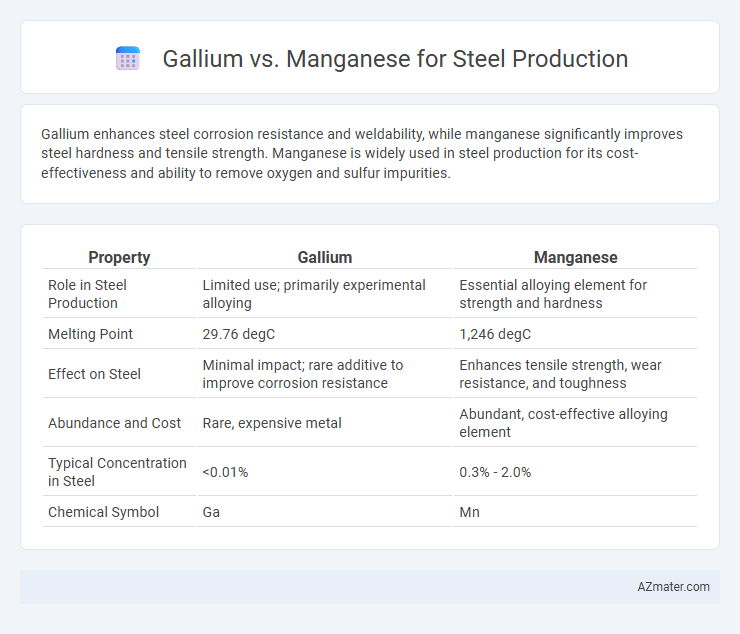
Gallium enhances steel corrosion resistance and weldability, while manganese significantly improves steel hardness and tensile strength. Manganese is widely used in steel production for its cost-effectiveness and ability to remove oxygen and sulfur impurities.
Table of Comparison
| Property | Gallium | Manganese |
|---|---|---|
| Role in Steel Production | Limited use; primarily experimental alloying | Essential alloying element for strength and hardness |
| Melting Point | 29.76 degC | 1,246 degC |
| Effect on Steel | Minimal impact; rare additive to improve corrosion resistance | Enhances tensile strength, wear resistance, and toughness |
| Abundance and Cost | Rare, expensive metal | Abundant, cost-effective alloying element |
| Typical Concentration in Steel | <0.01% | 0.3% - 2.0% |
| Chemical Symbol | Ga | Mn |
Introduction: The Role of Alloying Elements in Steel Production
Gallium and manganese serve distinct functions as alloying elements in steel production, influencing mechanical properties and corrosion resistance. Manganese is extensively used to improve hardness, tensile strength, and resistance to wear by deoxidizing and desulfurizing steel. Gallium, although less common, can refine grain structure and enhance specific steel applications through its unique metallurgical properties.
Chemical Properties: Gallium vs Manganese
Gallium exhibits a low melting point of 29.76degC and high corrosion resistance, making it useful in specialized steel alloys to improve machinability and resistance to oxidation. Manganese, a critical alloying element, has a high melting point of 1244degC and strong affinity for sulfur, enhancing steel's hardness, tensile strength, and wear resistance by reducing brittleness and promoting deoxidation. The chemical stability of gallium contrasts with manganese's reactivity, influencing their distinct roles in modifying steel microstructures and mechanical properties.
Abundance and Sourcing of Gallium and Manganese
Manganese is significantly more abundant in the Earth's crust, with an average concentration of about 0.1%, making it widely available and a cornerstone in steel production for its deoxidizing and alloying properties. Gallium, in contrast, is much rarer, primarily obtained as a byproduct of bauxite and zinc ore processing, with global production concentrated in a few countries such as China and Germany. The relative abundance and established mining infrastructure of manganese ensure consistent supply for steel manufacturing, whereas gallium's scarcity and extraction challenges limit its direct application in bulk steel production.
Effects on Steel Microstructure
Gallium in steel production influences the microstructure by refining grain boundaries, leading to improved toughness and resistance to cracking, whereas manganese primarily acts as a deoxidizer and desulfurizer, enhancing the steel's strength and wear resistance by stabilizing austenite phases. Gallium's presence can modify inclusions and promote uniform microstructural distribution, while manganese increases hardenability through the formation of manganese carbides. Optimizing the balance between gallium and manganese content results in steel with superior mechanical properties and enhanced thermal stability.
Influence on Mechanical Properties
Gallium in steel production enhances grain refinement and improves toughness by stabilizing the microstructure, leading to increased ductility and corrosion resistance. Manganese acts as a key deoxidizer and sulfide former, significantly strengthening steel and improving hardness and wear resistance by refining grain size and reducing brittleness. The combination of gallium and manganese optimizes mechanical properties by balancing strength, toughness, and ductility for advanced steel applications.
Corrosion Resistance Comparison
Gallium and manganese both influence steel corrosion resistance, but manganese is more commonly used as an alloying element to improve hardness and resistance to oxidation in steel. Gallium's effect on corrosion resistance is less significant and less studied, with minor impacts mainly due to its ability to form protective oxide layers. Steel alloys with higher manganese content generally exhibit better corrosion resistance in harsh environments compared to those with gallium additions.
Cost Implications in Industrial Scale
Gallium's high cost and limited availability make it impractical for large-scale steel production compared to manganese, which is abundant and economically feasible. Manganese acts as an effective alloying element improving strength and durability at a fraction of gallium's price, significantly reducing overall production costs. Industrial-scale steel manufacturers prefer manganese due to its cost-efficiency and proven performance in enhancing steel quality.
Environmental and Safety Considerations
Gallium in steel production offers low toxicity and minimal environmental impact due to its stable nature, whereas manganese extraction and processing often result in significant ecological hazards, including soil and water contamination. Manganese dust and fumes pose occupational health risks such as manganism, a neurodegenerative disorder, necessitating stringent safety measures. Gallium's limited use and lower toxicity profile make it a safer alternative, but the cost and availability constraints currently limit widespread adoption in steel manufacturing.
Technological Applications in Modern Steelmaking
Gallium enhances steel production by improving machinability and corrosion resistance in specialty steels, especially in electronic and aerospace components due to its unique semiconductor properties. Manganese remains essential for increasing tensile strength, toughness, and wear resistance in structural steels, stabilizing the steel's microstructure during the smelting process. Modern steelmaking integrates manganese for bulk strengthening applications while leveraging gallium's niche benefits in advanced alloy formulations for high-tech industrial applications.
Future Trends: Innovations and Research Directions
Research in steel production is increasingly focusing on gallium and manganese due to their distinct roles in improving steel properties; gallium shows promise in enhancing corrosion resistance and electrical conductivity, while manganese remains critical for strength and toughness. Emerging innovations explore gallium's potential as a dopant in advanced steel alloys to develop lightweight, high-performance materials for aerospace and automotive industries. Future trends emphasize sustainable extraction methods and alloy optimization through computational modeling to balance cost-effectiveness with superior mechanical and chemical performance.
Infographic: Gallium vs Manganese for Steel Production
 azmater.com
azmater.com